女性盆腔瘘管:诊断测试和管理
WHEC实践公报和临床管理指南为医疗服务提供者。教育补助金的妇女的健康和教育中心(WHEC)
Of all the non-fatal complications of obstetrics and gynecological surgery, fistula is the one we all fear the most. The word "fistula" comes from the Latin word meaning "pipe" or "tube" and is defined as an abnormal communication between epithelial or mesothelial surfaces. Pelvic fistulae are an abnormal communication among the genitourinary tract, the gastrointestinal tract, and the vagina or perineum. Genital tract fistulae have been described in the medical literature for the past several thousand years. Advancements in both the diagnosis and treatment of vaginal fistulae have been obtained over the past century as surgical interventions have become safer and surgical techniques have improved. The most common cause of fistulae worldwide is obstructed labor in the developing world. In developed countries, fistulae most commonly occur after benign gynecologic surgery, but obstructed labor, malignancy, radiation exposure, and inflammatory bowel disease can also cause fistulae. Fistulae significantly affect quality of life.
The purpose of this document is to discuss diagnostic studies and radiologic imaging which can help aid the diagnosis, but a thorough physical examination is the most important component in the evaluation and diagnosis of a fistula. Temporizing treatments are available to help ease patient suffering until surgical management can be performed. Surgical repairs can be performed using an abdominal, vaginal, or trans-anal approach. Although technically challenging, surgical repair is usually successful, but closure of the fistula tract does not guarantee continence of urine or feces, because there is often underlying damage to the bowel and bladder.
Incidence and Prevalence
In developed countries, the incidence of fistula formation after hysterectomy has been estimated at 0.1% with vesicovaginal fistulae occurring in 1 out of 455 to 1,800 hysterectomies (1). In a population study of 62,379 hysterectomies the incidence of vesicovaginal fistula was 1 in 455 with laparoscopic hysterectomy, 1 in 958 after abdominal hysterectomies, and 1 in 5,636 after vaginal hysterectomy (2).
Obstructed labor is a common cause of fistula formation in developing countries with incidence rates ranging from 0.1 to 5.39 per 1,000 deliveries and prevalence rates from 0 to 81.0 per 1,000 deliveries in Africa and Southern Asia (3). Because of improved prenatal care including monitored labor and access to cesarean delivery, fistulae caused by obstructed labor are now uncommon in the United States. Nonetheless, fistulae still occur after severe perineal lacerations, cesarean deliveries, peripartum hysterectomy, dilation and curettage for retained products of conception, and uterine rupture (3).
Obstetric uterine ruptures are uncommon, but 22% of these have been associated with concomitant bladder injury, which may rarely lead to vesicouterine fistulae (4). Vesicouterine fistulae account for only 1-4% of all genitourinary fistulae, with more than 90% of these caused by benign gynecologic surgery (5). Cesarean deliveries account for the majority of these, but additional causes include malignancy, radiation, chronic infections such as tuberculosis, or even ischemic necrosis from an intrauterine device (6). A different type of fistula, vesicocervical, is rare but has been reported after cerclage placement (6).
Urethrovaginal fistulae are also rare. Estimates of incidence are lacking; however, these may have increased since the adoption of synthetic midurethral slings to treat stress urinary incontinence (SUI) (7). Urethrovaginal fistulae have been reported after surgical repair of urethral diverticulum with an incidence ranging from 0.9% to 5% (7). Ureterovaginal fistulae are also uncommon but are most likely caused by benign gynecologic surgery. The incidence of iatrogenic ureteral injury during major gynecologic surgery is estimated at 0.5 - 2.5% with few of these resulting in fistula formation (8).
The majority (88%) of rectovaginal fistulae are caused by childbirth trauma (9). Diverticulitis accounts for approximately 66% of colovesical fistulae (10). Cervical, vaginal, and endometrial cancers account for 3-5% of vesicovaginal fistulae in industrialized countries, with radiation therapy reported to cause 1-2% of genitourinary or rectovaginal fistulae after treatment for cervical carcinoma (11).
Risk Factors
The majority of genitourinary fistulae in the United States are caused by injury at the time of hysterectomy. Postulated causes include blunt dissection trauma, thermal injury, or a portion of the bladder or ureter being clamped, incised or sutured to the vaginal cuff. Bladder injuries occur three times more often after abdominal compared with vaginal hysterectomy. Malignancy and radiation therapy can result in immediate or delayed fistula formation. Fistulae that occur during radiation therapy are usually caused by tumor necrosis. Fistulae that occur months to years after cancer treatment are either caused by recurrent malignancy or by capillary endothelial injury from radiation, which can result in poor tissue transfusion, ischemic necrosis, and tissue sloughing.
The incidence of fistula formation is directly proportional to the total dose of radiation given; healthy vaginal tissue can tolerate radiation doses up to 8,000 rads (12). A case study series of 207 women with vesicovaginal fistulae reported that 83% were associated with abdominal hysterectomy, 8% with vaginal hysterectomy, and 4% with radiation injury (13). Pressure necrosis from chronic indwelling Foley catheters, neglected pessaries, or retained sex toys have also been reported to cause fistula formation (14).
Pressure necrosis from obstructed labor is the most common cause of fistula formation in developing countries where women have limited access to perinatal care. These fistulae tend to be large, often including portions of both genitourinary tract and rectum.
Rectovaginal fistulae also can result from inflammatory bowel disease, diverticulitis, trauma, episiotomy, and perianal abscess formation such as cryptoglandular sepsis or hidradenitis suppurativa (15). These inflammatory rectovaginal fistulae often are associated with pain as a result of abscess formation and referred to as fistula-in-ano.
Additional risk factors for fistula development include cesarean delivery; endometriosis; traumatic injury to the pelvis; pelvic inflammatory disease; surgeries including pelvic, percutaneous, retroperitoneal and vascular surgery; underlying medical conditions that inhibit surgical healing such as diabetes, chronic steroid use, malignancy, vasculopathy, and tobacco use; and perioperative hematoma or infection (7),(15),(16). Despite these previously mentioned risk factors, approximately 30% of patients who develop fistulae will not have an identifiable risk factor (17).
Prevention
Strategies for preventing fistula formation are related to addressing the underlying causes. Intraoperative recognition and repair of genitourinary trauma may decrease fistula formation, and detection, and detection is improved by concomitant cystoscopy. Please review, Urinary Tract Injuries: Prevention & Management; available @ http://www.womenshealthsection.com/content/urog/urog020.php3
Clinical Presentation
The most common symptom in women with genitourinary fistulae is continuous urinary leakage. Patients often report leaking large amounts of urine when going from a recumbent to sitting or standing position. Patients may also report recurrent cystitis, pyelonephritis, fever, hematuria, perineal dermatitis, vaginal fungal infections, or pelvic pain. Postoperative genitourinary fistulae typically present with urinary leakage within 10 days of surgery, coinciding with removal of the Foley catheter. For patients with genitourinary fistulae, the amount of urinary leakage varies considerably depending on the size and location of the fistula. Proximal urethrovaginal fistulae often behave like vesicovaginal fistulae, with continuous leakage, fistulae at the level of the midurethra may mimic symptoms of stress urinary incontinence because of a shortened functional urethra, and patients with distal urethrovaginal fistulae may report spraying with urination or may be asymptomatic (18).
Vesicouterine fistulae may present with urinary continence, infertility, and amenorrhea or cyclic hematuria. This constellation of symptoms was first described by Youssef in 1957 in a patient who developed a vesicouterine fistula after a cesarean delivery and are sometimes called Youssef's syndrome. Vesicouterine fistulae may be associated with complete continence, intermittent leakage, or continuous leakage depending on the location of the fistula and the ability of the cervix to maintain continence (19).
Patients with rectovaginal fistulae may report vaginal passage of air, stool, purulent drainage or malodorous discharge (20). Digital rectal examinations are often normal, for evaluation of suspected rectovaginal fistulae caused by acute trauma, the abdomen, back and perineum can be examined to evaluate for external injury such as penetrating wounds or ecchymosis; pelvic fractures are frequently associated with rectal injuries. Approximately 70% of patients with colovesical fistulae report pneumaturia, and 50% report fecaluria because of connection between the urinary and colorectal systems (20).
Fistulae significantly affect the overall quality of life, healthy, sexuality and emotional well-being of women who develop them. Preoperative counseling that fistulae may rarely occur with surgery and frequent follow-up visits with candid, honest discussions if a fistula does occur, may help alleviate tension in both parties.
Diagnosis
To identify the presence of a fistula, a complete medical history and a through physical examination should be performed. Diagnostic imaging and endoscopy can often be helpful to distinguish urinary fistulae symptoms from other causes of urinary or anal incontinence.
Physical examination: Patients are most commonly placed in a dorsal lithotomy position to optimize visualization of the perineum and vagina, although left-lateral, prone jackknife, or genupectoral positions can also be used. The vulva and perineum are inspected; the external genitalia may be wet from urine or soiled with feces with associated skin irritation and erythema. Chronic fistulae may demonstrate dermatitis, vitiligo, and greenish gray vulvar encrustations, which are crystal precipitates created by urea-splitting vaginal flora (21).
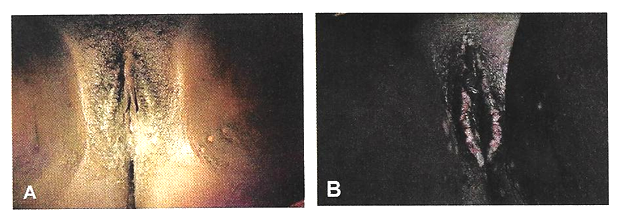
Figure 1. Vulvar changes with chronic exposure to urine. A and B show incontinence-associated dermatitis in two different patients. Patient B exhibits more pronounced vulvar encrustations.
A transparent plastic speculum may allow visualization of the fistula through the speculum. If a fistula is identified, a thorough examination should be completed, because approximately 15% of patients with one fistula will have multiple fistulae; in one case series of 43 patients with vesicovaginal fistulae, five (12%) were found to have a concurrent ureterovaginal fistula; a separate case series of 53 patients with urethrovaginal fistulae found that 10 (19%) had a separate vesicovaginal fistula (22).
Classification systems have been proposed to characterize genitourinary fistulae by size, location, and complexity (23). However, these systems are not commonly used by gynecologists in industrialized countries because of the heterogeneity of fistula types. Many surgeons simply document the type, number, and size of each identified fistula. Another option is to classify fistulae as simple or complex. In some systems, vesicovaginal fistulae are considered complex if they are larger than 1 cm in size, occur after radiation therapy , or recur after attempted fistula repair; rectovaginal fistulae are rated as simple if they are less than 2.5 cm in size, occur in the distal third of the vaginal, and are not associated with underlying bowel disease or radiation therapy.
With rectovaginal fistulae, it is also important to document whether the fistula occurs in the upper, middle or lower vagina. Rectovaginal fistulae that occur in the upper vagina typically are approached abdominally, whereas fistulae in the middle or distal vagina can be approached vaginally.
Finding ways of providing fistula repair services efficiently and cost-effectively, without comprising surgical outcomes and the overall health of the patient, is paramount. The Special Programme of Research, Development and Research Training in Human Reproduction of United Nations Development Program (UNDP), UNFPA, WHO and the World Bank, jointly with Engender Health, is conducting a facility-based multi-centre randomized controlled trial in some African countries to examine whether short-term (7-day) catheterization following surgical repair of "simple" fistula cases is inferior to longer-term (14-day) catheterization in terms of fistula repair breakdown.
Diagnostic Tests
Urinary Tract Fistulae
Cystourethroscopy should be performed during the evaluation of most genitourinary fistulae. This allows quantification of the number, size, and location of each fistula, including proximity to the ureters and the urethra. Permanent suture or mesh may be identified, which is helpful for surgical planning. Large bladder fistulae impair adequate bladder distention but may be successfully occluded with vaginal packing, a sponge on a stick, or the surgeon's finger. If bladder distention with fluid is not possible, air cystoscopy is an option (24). Immature fistulae often have a bullous edema appearance, and mature fistulae have smooth margins amidst bladder scarring.
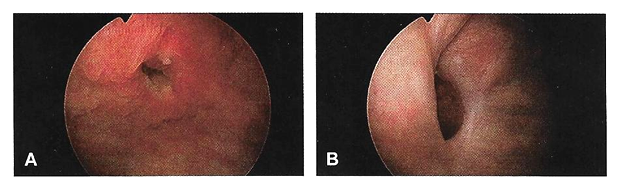
Figure 2. A. Immature vesicovaginal fistula; B. Mature vesicovaginal fistula.
Various tests for the diagnosis of urinary tract fistulae are summarized below:
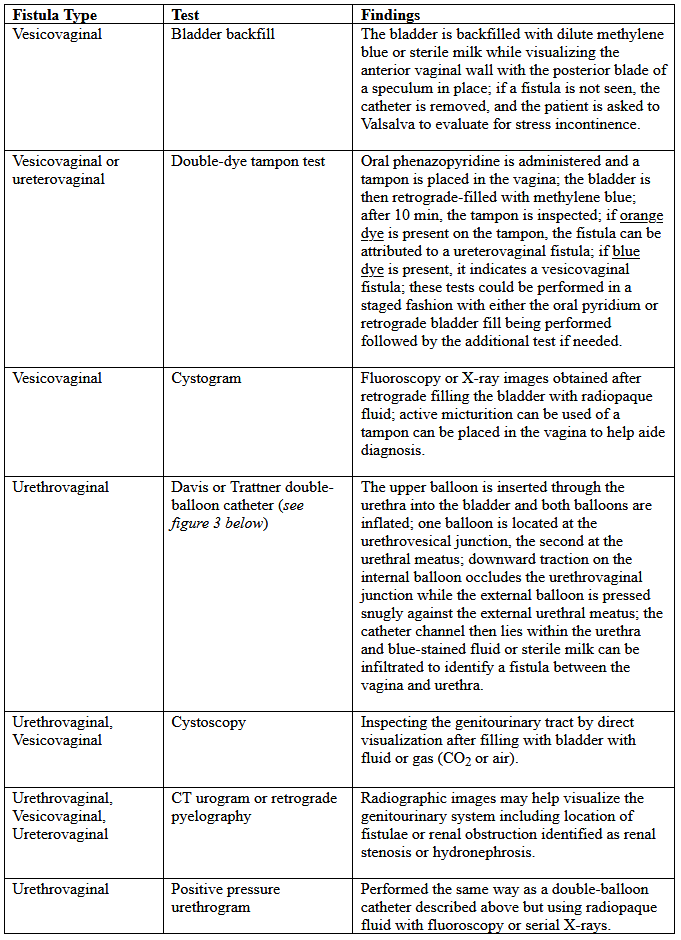
Table 1. CT: computed tomography; MRI: magnetic resonance imaging.
Cystoscopy can provide information about the upper urinary tract, because up to 12% of vesicovaginal fistulae will have a concomitant ureteral injury or ureterovaginal fistula (25). Historically, intravenous indigotindisulfonate sodium (indigo carmine) is administered before cystoscopy. Other options to help visualize ureteral reflux during cystoscopy include preoperative oral phenazopyridine, intravenous methylene blue or sodium fluorescein, or retrograde filling the bladder with fluids such as dilute methylene blue, dextrose, sterile milk or water. If cystoscopic ureteral efflux is still in doubt, intraoperative retrograde ureterography can be performed.
Urethrovaginal fistulae can be visualized by performing a positive pressure urethrogram using Davis or Trattner catheter with contrast material and fluoroscopy, MRI, CT or ultrasonography can be useful in the work up and diagnosis of fistulae in select situations, but when to use these additional modalities is left up to the discretion of the ordering physician.
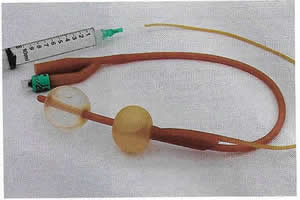
Figure 3. Trattner catheter.
Preoperative urodynamic evaluation is not routinely indicated in the evaluation of genitourinary fistulae. However, urodynamic evaluation can be useful to document the presence of pre-existent abnormal bladder function including urinary SUI or urgency urinary incontinence.
Bowel Injuries and Fistulae
Colonoscopy is indicated for many rectovaginal fistulae to evaluate for underlying inflammatory bowel disease or carcinoma. Ultrasonography and endoanal MRI can also be useful for diagnosis; however, ultrasonography may be less accurate than MRI because of inherent low soft tissue contrast (26). In addition to identification of endoanal fistula MRI can demonstrate perianal fistulae, rectovaginal septal abscesses, and defects in the anal sphincter complex.
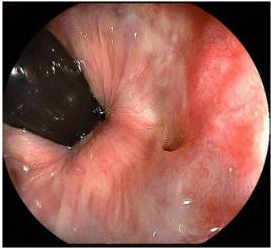
Figure 4. Rectovaginal fistula seen in colonoscopy.
Various tests for the diagnosis are summarized below:
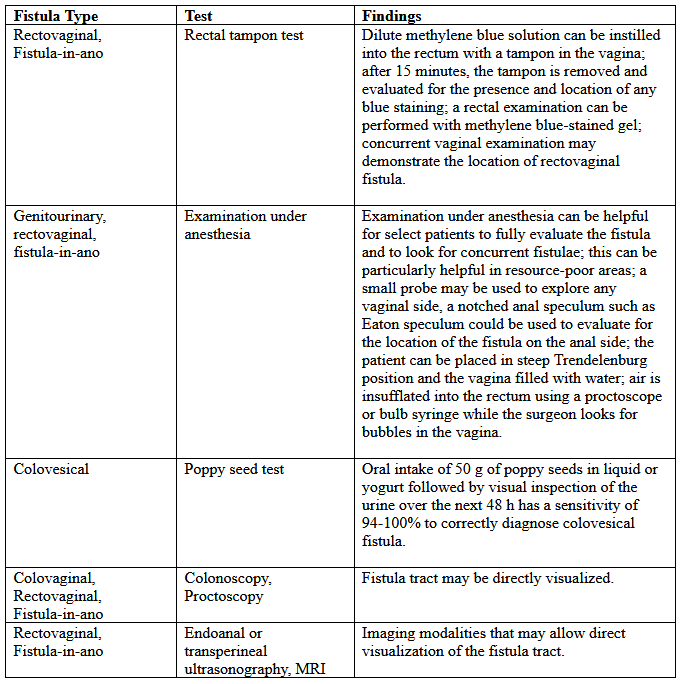
Table 2. MRI: magnetic resonance imaging.
Preoperative Care
Controlling urine and bowel leakage may minimize skin breakdown and psychological distress. Continuous leakage causes incontinence-associated dermatitis, which is sometimes referred to as perineal dermatitis (27). For vesicovaginal fistulae, an indwelling catheter may help prevent or decrease perineal dermatitis and may allow for spontaneous closure of the fistula when the fistula first forms. Topical barrier products such as petroleum, zinc oxide, and dimethicone-based ointments can form and occlusive barrier to prevent skin damage. Many patients use a variety of incontinence products such as pads, diapers, and protective bed sheets to help manage their symptoms. Frequent pad changes are required to minimize irritation.
An incontinence-collecting device can be created for apical genitourinary fistulae to decrease perineal irritation. This can be done by cutting a hole in a contraceptive diaphragm or menstrual cup with a 5-mm punch biopsy and then inserting a Pezzer or Malecot catheter (See figure 5 below). The diaphragm or cup traps urine and diverts it to a collecting leg bag protecting the external genitalia from breakdown. The catheter can be worn for months if needed until a repair is performed.
Preoperative vaginal estrogen may help induce spontaneous closure of the fistula in postmenopausal patients provided there are no contraindications to its use. Case reports for the treatment of genitourinary and rectovaginal fistulae caused by neglected pessaries commonly include the use of estrogen as part of fistula management (28).

Figure 5. Contraceptive diaphragm with a Malecot catheter.
Conservative Management
Prolonged Foley drainage for 4-6 weeks may allow spontaneous closure in approximately 7% to 15% of patients with vesicovaginal fistulae and 5% of patients with vesicouterine fistulae (1),(5). Prolonged catheterization has been combined with electrocoagulation of the fistula tract. This approach is most likely to be successful in small fistulae, and there is a risk of enlarging the fistula if the tissue is over-cauterized. A non-comparative case series of 15 patients with vesicovaginal fistulae less than 3.5 mm treated with cautery reported cure in 73% (29). Another case series reported 100% cure in 6 patients with vesicovaginal fistulae less than 3 mm treated with cautery and catheter drainage for 10 days (28). Cystoscopic laser fulguration also has been reported to treat small fistulae in a similar fashion as electrocautery (30).
Fibrin glue and collagen plugs also have been reported for the treatment of rectovaginal and ano-rectal fistulae, with success rates between 11% and 70% (31). Setons are another minimally invasive treatment option. A seton is a foreign body such as a suture of vessel loop that can be passed through the fistula tract. If left loose, the fistula tract will mature; if tightened, the body will slowly extrude it, with the tissue healing as it is expelled (32). A systematic review of 18 studies demonstrated successful fistula closure for fistula-in-ano with seton use in 95 - 97% of 448 patients; fecal incontinence was common in this cohort after fistula management, with 25% reporting fecal incontinence if the seton passed through the internal anal sphincter and 5% if the internal anal sphincter was spared (33). A French prospective cohort study of 79 patients with rectovaginal fistulae reported successful closure in 5% when only a seton was used (32),(33).
One-third to one-half of patients with rectovaginal fistulae are asymptomatic or have minimal symptoms, may require no treatment, especially present with Crohn's disease (34). Rectovaginal fistula is a rare, but dreaded complication of Crohn's disease that is exceedingly difficult to manage. Treatment algorithms range from observation and medical therapy to local surgical repair and proctectomy. The key to successful management of these fistulae therefore rests on a multidisciplinary approach between the patient, gastroenterologists, and surgeons, with open communication about expectations and goals of care.
Surgical Management
In view of the observations made while dealing with different pelvic fistulae along with review of some recent literature following comments are being made for the operating team. All the members of operating team should be:
- Well conversant with normal and morbid anatomy of pelvis;
- Familiar with different surgical approaches and techniques;
- Aware of all relevant urological procedures on bladder, urethra and ureters;
- Expert in harvesting of need based reinforcement (interposition or on-lay) flaps;
- Have an updated knowledge of preventing pelvic fistulae under adverse circumstances like repeat cesarean sections, subtotal hysterectomy for control of bleeding, multiple large fibroids distorting pelvic anatomy and frozen pelvis due to sepsis / endometriosis etc.
In the end remember that vast majority of fistulae are preventable. Prevention of such fistulae, include antenatal education, awareness of women in their reproduction periods, easily available transport facilities and widespread availability of obstetrical facilities even in remote rural areas. The fundamental principles of repair are adequate exposure; tension free approximation of fistula edges; non-overlapping suture lines; multilayered closure of bladder and vagina at right angle to each other; good hemostasis and adequate postoperative bladder drainage can be achieved through both vaginal and abdominal route. However, when fistula is complex, vaginal exposure of fistula is suboptimal which may compromise the repair or endanger the ureters. In these cases, a transabdominal approach should be preferred. Nowadays all repairs are mandatory to be strengthened with routine use of reinforcement flaps, for which number of such flaps are described in literature with their multifactorial mechanism of action. Omental flap in transperitoneal and Martius flap in transvaginal approach are two such reinforcement flaps, which are most versatile and vascular, and can be harvested with ease without producing any functional or cosmetic donor site deformities (35).
Catheter Management after Pelvic Reconstructive Surgery
Voiding dysfunction complicates 21 - 42% of pelvic reconstructive procedures (38). Patients with failed voiding trials after pelvic reconstructive surgery are commonly discharged home with temporary catheterization until a repeat voiding trial is performed in the office. Although catheterization is short in duration, it is a large stressor for patients, and is often considered the worst part of the surgical experience and even a surgical complication.
Traditionally, transurethral catheters continuously drain into a bag and are associated with increased rates of urinary tract infection and patient discomfort and decreased patient satisfaction. Alternates include suprapubic catheters, clean intermittent self-catheterization, and catheter valves. Suprapubic catheters and clean intermittent self-catheterization have lower infection risks and are more preferred by patients compared with transurethral catheters. Clean intermittent self-catheterization requires adequate manual dexterity to perform; and suprapubic catheters require surgical placement. Catheter valves were introduced in 1986, marketed to help maintain bladder tone and capacity, and linked to decreased rates of urinary tract infections and catheter encrustation with increased patient satisfaction.
A variation in management is manual intermittent catheter plugging and unplugging (plug-unplug). The transurethral catheter is occluded with a plastic plug and unplugged to empty the bladder. Plug-unplug catheters allow patients to intermittently drain the bladder without a leg bag that potentially limits mobility and performance of activities of daily living. This study compared effects on activity between two catheter management systems after failed voiding trial after pelvic reconstructive surgery (39). The conclusion was postoperative activity does not differ in patients discharged with plug-unplug or continuous drainage catheters, but those with plug-unplug perceive easier management and ability to complete activities of daily living. The plug-unplug method is an acceptable alternative to traditional catheterization after pelvic reconstructive surgery.
Managing Vesicovaginal Fistulae
http://www.womenshealthsection.com/content/urogvvf/urogvvf002.php3
Surgical Management of Lower Urinary Tract Fistulae
http://www.womenshealthsection.com/content/urogvvf/urogvvf007.php3
Rectovaginal Fistulae and Fecal Incontinence
http://www.womenshealthsection.com/content/urogvvf/urogvvf008.php3
Sexual Function after Fistula Repair
Sexual activity and function should be determined before initiating fistula treatment, including a discussion of the presence or absence of dyspareunia before fistula formation. After surgical repair, many surgeons recommend abstaining from sexual intercourse for 6-12 weeks. Postoperative sexual function was evaluated in 99 women 6 months after vaginal or abdominal surgical repair of genitourinary fistula (1). The surgical repair was chosen at the discretion of the surgeon based on fistula type and location. Sexual function was evaluated using the Female Sexual Function Index, a validated questionnaire assessing domains of desire, arousal, lubrication, orgasm, satisfaction, and pain. Women reported significant improvement in sexual function after Latzko transvaginal or transabdominal fistula repairs with no statistically significant differences in Female Sexual Function Index scores.
When assessment of sexual function is a secondary endpoint and subject burden related to questionnaire length is a priority, the 9-item Female Sexual Function Index may provide important information about sexual function in English-speaking peri- and post-menopausal women (36). A separate study evaluated sexual function in patients who had a Martius flap for treatment of vesicovaginal fistulae or bladder outlet obstruction (37). This study also evaluated sexual function using the Female Sexual Function Index, higher scores indicate better sexual function with a maximum score of 36. There were no differences seen between groups with an average Female Sexual Function Index score of 28.
Summary
The majority of fistulae encountered in the industrialized world are unexpected and result in significant physical and psychological discomfort. A successful repair will not be possible for some patients such as those with small bladder capacity or poor rectal compliance. Urinary or fecal diversion procedures are available and may restore continence in these cases. Nevertheless, most patients can have successful closure of the fistula tract with improved or restored long-term quality of life and continence. Surgical correction of pelvic fistulae is still a great challenge and requires a team approach for better results. Such team must be well conversed with surgical anatomy and different procedures on various pelvic organs. We recommend transabdominal approach for complex fistulae, which also allows simultaneous correction of other morbidities and harvesting of different reinforcement flaps as per requirement is almost mandatory for favorable outcome.
References:
- Mohr S, Brandner D, Mueller MD, et al. Sexual function after vaginal and abdominal fistula repair. Am J Obstet Gynecol 2014;211:74.e1-6
- Walter MD, Karram MM. Urogynecology and reconstructive pelvic surgery. 3rd ed. Philadelphia (PA): Mosby; 2007
- Rogers RG, Jeppson PC. Current diagnosis and management of pelvic fistulae in women. Obstet Gynecol 2016;128:635-650
- Wong MG, Wong K, Rezvan A, et al. Urogenital fistula. Female Pelvic Med Reconstr Sug 2012;18:71-78
- Cichowski S, Rogers RG. Nonsurgical management of a rectovaginal fistula caused by a Gellhorn pessary. Obstet Gynecol 2013;122:446-449
- Singh RB, Dalal S, Nanda S, Pavithran NM. Management of female uro-genital fistulas: Framing certain guidelines. Urol Ann 2010;2(1):2-6
- Rock JA, Jones HW III. Te Linde's operative gynecology. 9th ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2003
- Likic IS, Kadija S, Ladjevic NG, et al. Analysis of urologic complications after radical hysterectomy. Am J Obstet Gynecol 2008;199:644.e1-3
- Jacob TH, Perakath B, Keighley MR. Surgical intervention for anorectal fistula. The Cochrane Database of systematic Reviews 2010. Issue 5. Art. No: CD006319. DOI: 10.1002/14651858. CD006319.pub2
- Melchoir D, Cudovic D, Jones J, et al. Diagnosis and surgical management of colovesical fistulae due to sigmoid diverticulitis. J Urol 2009;182:978-982
- Pshak T, Nikolavsky D, Terlecki R, Flynn BJ. Is tissue interposition always necessary in transvaginal repair of benign, recurrent vesicovaginal fistulae? Urology 2013;82:707-712
- Eswara JR, Raup VT, Heningburg AM, Brandes SB. Pelvic radiation is associated with urinary fistulae repair failure and need for permanent urinary diversion. Urology 2015;85(4):932-936
- Cowgill KD, Bishop J, Norgaard AK, et al. Obstetric-fistula in low-resource countries: an under-valued and under-studied problem-systematic review of its incidence, prevalence, and association with stillbirth. BMC Pregnancy Childbirth 2015;15:193
- Aminsharifi A, Afsar F, Shirazi MK. A rectovesical approach for the laparoscopic repair of vesicouterine fistulas. Int J Gynaecol Obstet 2014;124:148-150
- Kleeman SD, Vasallo B, Segal J, Karram MM. Vesicocervical fistula following insertion of a modified McDonald suture. BJOG 2002;109:1408-1409
- Blavivas JG, Mekel G. Management of urinary fistulas due to midurethral sling surgery. J Urol 2014;192:1137-1142
- Gouttgens KW, Smeets RR, Stassen LP, et al. The disappointing quality of published studies on operative techniques for rectovaginal fistulas: a blueprint for a prospective multi-institutional study. Dis Colon Rectum 2014;57:888-898
- Donaldson JF, Tait C, Rad M, et al. Obstructive uropathy and vesicovaginal fistula secondary to a retained sex toy in the vaginal. J Sex Med 2014;11:2595-2600
- Biswas A, Bal R, Alauddin M, et al. Genital fistula - our experience. J Indian Med Assoc 2007;105(3):123-126
- Fazio VW, Church JM, Delaney CP, editors. Current therapy in colon and rectal surgery. 2nd ed. Philadelphia (PA); Mosby; 2005
- Singh RB, Pvithran NM, Khatri HL, Nanda S. Technical aspects in the management of complex vesicovaginal fistulae. Trop Doct2005;35(1):40-41
- Howard D, Delancy JO, Burney RE. Fistula-in-ano after episiotomy. Obstet Gynecol 1999;93:800-802
- Goh JT. A new classification for female-genital tract fistula. Aust N Z J Obstet Gynecol 2004;44:502-504
- Schultheiss D, Machtens SA, Jonas U. Air cystoscopy: the history of an endoscopic technique from the late 19th century. BJU Int 1999;83:571-577
- Doyle PJ, Lipetaskaia I, Duecy E, et al. Sodium fluorescein use during intraoperative cystoscopy. Obstet Gynecol 2015;125:548-550
- Alkatib AA, Santoro GA, Gorgun E. Tandem vaginoscopy with colonoscopy: a diagnostic technique for the assessment of colovaginal fistula. Colorectal Dis 2016;18:483-487
- Gray M. Optimal management of incontinence-associated dermatitis in the elderly. Am J Clin Dermatol 2010;11:201-210
- Arias BE, Ridgeway B, Barber MD. Complications of neglected vaginal pessaries: case presentation and literature review. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:1173-1178
- Rosenblatt P, Adams SR, Adelowo A. An improved urinary drainage device for management of vesicovaginal fistula. Female Pelvic Med Reconstr Surg 2012;18(5 suppl):S95
- Dogra PN, Nabi G. Laser welding of vesicovaginal fistula. Int Urogynecol J Pelvic Floor Dysfunct 2001;12:69-70
- Shrivan MK, Alamdari DH, Ghoreifi A. A novel method for iatrogenic vesicovaginal fistula treatment: autologous platelet rich plasma injection and platelet rich fibrin glue interposition. J Urol 2013;189:2125-2129
- Corte H, Maggiori L, Treton X, et al. Rectovaginal fistula: what is the optimal strategy? An analysis of 79 patients undergoing 286 procedures. Ann Surg 2015;262:855-860
- Vial M, Pares D, Pera M, Grande L. Faecal incontinence after seton treatment for anal fistulae with and without surgical division of internal and anal sphincter: a systematic review. Colorectal Dis 2010;12:172-178
- DeLeon MF, Hull TL. Treatment strategies in Crohn's-associated rectovaginal fistulae. Clin Colon Rectal Surg 2019;32:261-267
- Kapoor R, Ansari MS, Singh P, et al. Management of vesicovaginal fistula: An experience of 52 cases with a rationalized algorithm for choosing the transvaginal or transabdominal approach. Indian J Urol 2007;23:372-376
- Carpenter JS, Jones SM, Studts CR, et al. Female Sexual Function Index short version: A MsFLASH item response analysis. Arch Sex Behav 2016;45(8):1897-1905
- Lee D, Dillon BE, Zimmern PE. Long-term morbidity of Martius labial fad pad graft in vaginal reconstruction surgery. Urology 2013;82:1261-1266
- Kandadai P, Duenas-Garcia OF, Pilzeck AL, et al. A randomized controlled trial of patient-controlled valve catheter and indwelling Foley catheter for short-term bladder drainage. Female Pelvic Med Reconstr Surg 2016;22:88-92
- Boyd SS, O'Sullivan DM, Tunitsky-Bitton. A comparison of two methods of catheter management after pelvic reconstructive surgery. Obstet Gynecol 2019;134:1037-1045
发布时间: 10 December 2019
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


