Understanding Overactive Bladder (OAB) In Women
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
The term Overactive Bladder (OAB) encompasses urinary symptoms such as frequency, urgency, and urge incontinence. This common medical condition is seen in roughly one in five women aged 25-64. The purpose of this document is to help us understand neurology and physiology of overactive bladder and to formulate the best treatment for the patient. Urge incontinence has a much more dramatic impact on a woman's quality of life than stress incontinence, because stress incontinence is predictable and controllable. In contrast, urge incontinence manifests as an unpredictable, involuntary void in which urine is released in a gushing stream, often in quantities large enough to soak through heavy absorbent pads. Since therapies of stress incontinence and urge incontinence is completely different, the evaluation of incontinence is very important.
In aging women, the prevalence of frequency, urgency, and urge incontinence is much higher than that of stress incontinence. Among women 60 to 80 years of age- growth, the largest segment of our population - as many as 50% experience frequency, urgency and urge incontinence. The tremendous expense of urinary incontinence is increasingly recognized. In the United States the direct costs associated with incontinence is estimated at $ 26 Billion per year and approximately 17 million US adults have OAB (1). Medical consequences of OAB can be quite serious: Patients may develop complications such as decubitus ulcers, perineal rashes, cystitis, urinary tract infections, urosepsis, and renal failure. Women with OAB are also at considerable risk for falls and fractures - probably because they are frequently in a hurry to reach the bathroom.
Contributing factors and causes of overactive bladder:
A potential etiology for OAB is neurological dysfunction stemming from disease or aging and resulting in disruption of the complex control system present in the lower urinary tract. It is thought to be multifactorial. Coexisting conditions may also contribute to symptoms or may even be the sole cause. Examples include:
- Injury or diseases of the nervous system: At a local level, urge incontinence can develop secondary to intrinsic detrusor myogenic abnormalities. It disrupts voluntary control of voiding in adults, triggering the reemergence of reflex voiding, which leads to bladder hyperactivity and urge incontinence.
- Outlet obstruction: Urethral obstruction or foreign body in the bladder and in men with benign prostatic hyperplasia can result in urge and frequency of micturition.
- Urinary tract infection or cystitis: Urinary tract infection without associated neurologic or obstructive disorders can resolve after 3 days of antibiotic therapy (course treatment will continue for 1 to 2 weeks). It can resolve the symptoms of urge and frequency.
- Detrusor sphincter dysnergia: Most commonly secondary to spinal cord injury or multiple sclerosis, may affect younger men and women.
- A deficient urethral sphincter: In women with stress incontinence, urine leakage into the urethra stimulates urethral afferents that can induce involuntary voiding reflexes.
- Urogenital atrophy: Irritative symptoms of lower urinary tract in the form of frequency, urgency, and dysuria can result from lack of estrogen, leading to urogenital atrophy.
- Pelvic organ prolapse: It is another common coexisting condition. Although the correlation between anatomic descent of pelvic organs and lower urinary tract symptoms is poorly understood, frequency and urgency -with or without urge incontinence -coexist with symptomatic pelvic organ prolapse in approximately 30% to 50% of cases.
- Enlarged uterus or adnexal mass: It may cause external compression of the bladder and lead to lower urinary tract symptoms.
- Previous surgery: The anterior vaginal wall or bladder neck repairs may sometimes trigger de novo symptoms of frequency, urgency, and urge incontinence. In women who have undergone a previous anti-incontinence procedure, these symptoms may be related to some form of outlet obstruction.
Framework for understanding bladder health:
Bladder relaxation and contraction: An interplay of nerve impulses
Source: OBG Management; December 2003; Image: Birck Cox
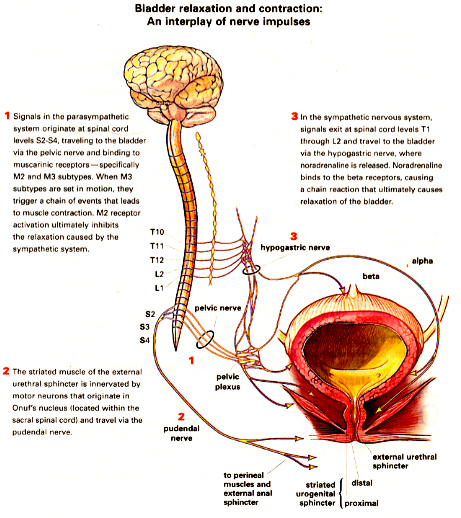
Diagnosis:
More specific questions can help to identify the nature of the incontinence, and queries about whether or not patients use pads or other protective devices may also encourage further dialogue. Affirmative answers to direct questions about urine loss associated with an urge to urinate or postvoid dribbling may be suggestive of detrusor instability, the most common cause of OAB symptoms. Discuss about duration and characteristic of urine loss and evaluate severity of the condition; type and number of pads or briefs used daily or weekly, number of incontinent voids and changes in activities of daily living or fluid intake patterns. Query patients about presence of pain, hematuria and infection, and about changes in bowel habits and sexual function.
Review of past incontinence management strategies is important to formulate effective treatment plan for the patient. OAB is characterized by urgency, frequency and urge incontinence. Stress incontinence is associated with coughing and exercise. Mixed incontinence is the combination of stress/OAB features. Since these disorders can co-exist, comprehensive evaluation and therapy are absolutely essential. More accurate diagnosis has led to improvement in treatment outcomes and better patient selection for medical and behavioral therapy.
Urinary cytology and cystoscopy are recommended in patients with recurrent urinary tract infections and hematuria. Postvoid residual (PVR) should be measured in patients with suprapubic tenderness or distension, diabetes, or neurologic disease, as well as in those taking medications that can interfere with bladder emptying. Determining PVR volume requires catheterization or pelvic ultrasound. Residual volumes of less than 50 mL are generally considered indicative of adequate bladder emptying, whereas repeated residual volumes greater than or equal to 100 to 200 mL usually represent inadequate emptying (2).
Urodynamic testing is indicated in the elderly (>75 y), and in patients with an elevated PVR, mixed symptoms of stress and urge incontinence, or unsuccessful surgery for incontinence. These tests include the following: cystometry or urethrocystometry (tests of detrusor storage and contractile function), uroflowmetry or voiding pressure studies (tests of urine flow rate and mechanism), and urethral pressure profilometry (test of resting and dynamic pressures in the urethra). Such specialized tests help to determine the anatomic and functional status of the urethra and bladder, and may be useful in determining the cause of AOB (3).
Treatment:
Non-surgical treatments can be non-pharmacologic or pharmacologic. They can be used alone or in combination, depending on the severity of the condition and the patient's ability to comply with treatment.
- Non-pharmacologic Therapy
Pelvic floor exercise, Biofeedback, Bladder retraining: For details please review the following linkhttp://www.womenshealthsection.com/content/urog/urog002.php3
- Pharmacologic Therapy
Anticholinergic (antimuscarinic) agents treat OAB by limiting uncontrolled contractions of the detrusor muscle. The main problem with these agents is the side effects; dry mouth is the most frequent cause of discontinuation. For details please review the following link:http://www.womenshealthsection.com/content/urog/urog006.php3
Overactive Bladder (OAB): A Treatment Guide:
Patient may not report lower urinary tract symptoms, so clinicians should identify overactive bladder (OAB) through careful patient interview. Used together, behavioral modification and pharmacologic treatment are more effective than either approach alone. Five agents have been approved for pharmacologic treatment of OAB; all block the M3 receptor to prevent detrusor contraction with similar efficacy. The relative affinity of an antimuscarinic agent for each receptor subtype is responsible for the varying side-effect profiles of the drugs in this class. The greatest efficacy in treating OAB is achieved using combination therapy with behavioral modification and pharmacologic treatment with antimuscarinic agents (4). Behavior therapy begins with patient education about lifestyle changes that can prevent the exacerbation of symptoms and facilitate social functioning. Avoiding bladder irritants such as caffeine, alcohol, and high fluid load is critical. Developing strategies such as toilet mapping can help patients deal with the social aspects of their condition. Bladder training places the patient on a graduated voiding schedule in order to increase the capacity of the bladder and restore normal bladder function (5). Pelvic floor exercises, also known as Kegel exercises, are the mainstay of behavioral therapy, alleviating symptoms of urgency and incontinence. Such techniques help patients to suppress detrusor contractions and occlude the urethra when involuntary voiding is imminent. It is essential that these exercises are performed properly; therefore, verbal provider feed-back in response to vaginal palpation, or biofeedback with a physical therapist, should be used to ensure optimal results. There appears to be no advantage to biofeedback relative to verbal feedback, in terms of reducing incontinence (6).
Distinguishing among effective pharmacologic agents: Five pharmacologic therapies have been approved for the treatment of OAB -- oxybutynin, approved in immediate-release, extended release, and transdermal patch formulations; immediate- and extended-release tolterodine; trospium chloride; solifenacin; and darifenacin (7). Each of these agents is a muscarinic receptor antagonist, and all have demonstrated substantial efficacy in reducing urinary urgency, frequency, and urinary incontinence. The efficacy of these agents is a result of M3 receptor blockage at the level of the detrusor muscle. This mechanism of action, however, can also lead to adverse effects in 2 distinct ways. First, antimuscarinic agents block M3 receptors elsewhere in the body, leading to side effects such as dry mouth, which results from M3 blockade in salivary glands. M3 receptors can also be found in the brain, though their role is not clearly defined; within the gastrointestinal tract, where M3 receptors predominate and blockage theoretically could lead to blurry vision, although practically this is not often the case. M3 receptors also have been identified in the liver and the kidney. Second, the blockade of other muscarinic receptor subtypes leads to adverse effects of antimuscarinic therapy. M1, M2, M4 and M5 receptor subtypes are found throughout the body, playing many roles in normal physiologic function. Therefore, blockade of muscarinic receptor subtypes by nonspecific agents occurs in a myriad of organ systems, including the salivary glands, lacrimal glands, gallbladder, gastrointestinal tract, respiratory system and eye (4, 8).
The potential side effects vary widely from drug to drug, depending on its relative affinity for each muscarinic subtype. Only darifenacin has been determined to be highly selective for the M3 subtype, the receptor primarily involved in bladder contraction. The affinity of darifenacin for M3 is: 9-fold greater than its affinity for M1; 12-fold greater than its affinity for M5; at least 59-fold greater than its affinity for M2 and M4. Transdermal application (oxybutynin TDS) eliminates gastrointestinal effects on drug integrity and decreases hepatic first-pass metabolism of drugs. As a result, side effects such as dry mouth and constipation are reduced. Unfortunately, the transdermal formulation of oxybutynin results in application site pruritus in 16.8% of patient and application site erythema in 5.6% of patients.
Cardiovascular Risks of Anticholinergic Therapy: Two adverse effects of anticholinergic therapy are prolongation of the QT interval and elevation of heart rate, which may precipitate dangerous arrhythmias, such as torsades de pointes (TdeP), in susceptible patients. The QT effects of solifenacin, trospium chloride, and darifenacin have been studied in randomized, positively controlled clinical trials. Trospium chloride and darifenacin had no QT effect; the data in this clinical trial on solifenacin were inconclusive (9). No causal link between drug-induced tachycardia and adverse cardiac outcomes has been established, but patients with cardiac abnormalities should not be given drugs that elevate heart rate. Neither solifenacin nor darifenacin has a significant effect on heart rate compared with placebo. The cardiovascular adverse effects of non-cardiac drugs, such as QT interval prolongation and heart rate changes, are important to consider when using anticholinergic drugs to treat OAB symptoms, especially in older patients who have heart disease. The prolongation of QT interval caused by some agents in this class may lead to TdeP and sudden death. A causal link between drug-induced tachycardia and adverse cardiac outcomes has not been established; however, patients with cardiac abnormalities should not receive drugs that significantly increase heart rate if effective alternatives are available. In the end, the benefit of any intervention has to outweigh the risk. In this context, patients should not be deprived of the benefits of treatment for a debilitating disease like OAB if they can be treated with drugs that have been clearly demonstrated to be devoid of important and life-threatening cardiac toxicity.
Adverse Cognitive Effects of Anticholinergic Therapy: Memory impairment, increased reaction time, and sedation are adverse effects of anticholinergic therapy. The cognitive effect of pharmacologic therapy for overactive bladder is influenced by the patient's age, anticholinergic load, and physiology. The relative affinities of oxybutynin and darifenacin for the M1 receptor may explain differences in the cognitive effect of these drugs seen in clinical trials (10). Aging leads to increased permeability of the blood-brain barrier (BBB), decreased acetylcholine production, and a reduction in the number and distribution of muscarinic receptors in the CNS. The relationship between anticholinergic agents and these systems largely determines whether cognitive effects will occur. The BBB is the protective barrier that regulates passage of substances from the vascular system to the internal milieu of the CNS. It is composed of endothelial tight junctions and a variety of cell types and structures, including astrocytic foot processes. Passive diffusion of substances is limited and favors substances with the following properties: molecules smaller than 400 daltons, low polarity, and lipophilicity. The anticholinergic agents approved for treatment of OAB have varying abilities to permeate the BBB. Oxybutynin, which is lipophilic, of low molecular size, and neutrally charged, is the most likely to penetrate the BBB. The other agents are less likely to penetrate BBB due to their larger size, low lipophilicity, and polarity. In addition to having these beneficial characteristics, darifenacin is known to be a substrate of P-glycoprotein, a system by which substances are actively effluxed across the BBB back into the bloodstream (11).
Normal aging, however, has been shown to increase the permeability of the BBB. Consequently, even though many approved OAB agents do not normally cross the BBB, they may be more likely to reach CNS of older patients. The permeability of the BBB is also altered by some diseases, such as multiple sclerosis, diabetes, and dementia. Additionally, environmental factors including stress and heat can disrupt the BBB, resulting in greater permeability. Lastly, certain drugs themselves (eg, cholinesterase inhibitors) can alter the permeability of the BBB.
Discontinuation rates for anticholinergic medications are high regardless of the class of medication used. Clinical trials in general report higher medication-adherence rates in comparison with population-based studies. Large, population-based studies are required to determine the cause for poor adherence to anticholinergic medications in clinical practice. Our high discontinuation rates across anticholinergic drug classes also highlight the need for more effective therapies for lower urinary tract systems (12). Therefore, we must be vigilant regarding alternative forms of treatment (fluid modification, bladder training, and pelvic floor rehabilitation) for OAB and increase our awareness that this group of women is being treated inadequately.
Conclusion:
Overactive bladder (OAB) is a common medical condition that can erode a woman's psychological and social well-being, and may have serious health consequences if left untreated. Therapy may include non-pharmacologic techniques, medication or a combination. Usefulness of pharmacotherapy may be limited by adverse reactions such as dry mouth, although newer medications such as ER oxybutynin and tolterodine tartrate may have slightly fewer side effects. Appropriate early intervention, which includes identifying the disorder, is a key factor in slowing the progression of detrimental changes in the lower urinary tract.
References:
- Abrams P, Cardozo L, Fall M et al. The standardization of terminology of lower urinary tract function: report from the Standardization Sub-committee of the International Continence Society. Neurourol Urodyn 2002;21:167-178
- Stewart WF, Van Rooyen JB, Cundiff GW et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003;20:327-336
- Ouslander JG. Management of overactive bladder. N Engl J Med 2004;350:786-799
- Abrams P, Andersson KE, Buccafusco JJ et al. Muscarinic receptors: their distribution and function and function in body systems, and implications for treating overactive bladder. Br J Pharmacol 2006;148:565-578
- Borello-France D, Burgio KL. Nonsurgical treatment of urinary incontinence. Clin Obstet Gynecol 2004;47:70-82
- Burgio KL, Goode PS, Locher JL et al. Behavioral training with and without biofeedback in the treatment of urge incontinence in older women: a randomized controlled trial. JAMA 2002;288:2293-2299
- Diokno AC, Appell RA, Sank PK et al. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc 2003;78:687-695
- Iverson HA, Fox D III, Nadler LS et al. Identification and structural determination of the M (3) receptor sensitivity. Eur Urol Suppl 2005;280:24568-24575
- US Food and Drug Administration. Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs. Rockville, MD: US Department of Health and Human Services; October 2005.
- Ancelin ML, Artero S, Porter F et al. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ 2006;332:455-459
- Lewis JD, Schinnar R, Bilker WB et al. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemil Drug Saf 2007;16:393-401
- Gopal M, Haynes K, Bellamy SL et al. Discontinuation rates of anticholinergic medications used for the treatment of lower urinary tract symptoms. Obstet Gynecol 2008;112:1311-1318
Published: 9 February 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


