Urodynamic Assessment: ElectromyographyWHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC). The study of electrical potentials generated by the depolarization of muscle is electromyography (EMG). Electrical activity is produced by intracellular and intercellular neuronal activity. Within the nerve cell and its processes is semipermeable, lipoprotein, bilayered membranes with irregular distributions of ions on either side. In the patient presenting with urinary incontinence or a voiding disturbance, it is important to consider the possible neurologic causes that may be associated with the symptoms. When the history, physical examination, and urodynamic findings suggest the possibility of nervous system impairment, the adjunctive use of electrophysiologic testing is useful to determine whether neuropathy is a significant component of the patient's problem, and if it is, to localize the area of the central, peripheral, or autonomic nervous system chiefly affected. Thus, the older patient with urge incontinence and headaches, the younger patient with incontinence and difficulty voiding, the patient with low back pain and overflow incontinence, and the patient with stress urinary and anal incontinence may have the respective underlying disorders of brain tumor, multiple sclerosis, intervertebral disk disease, and pudendal neuropathy diagnosed and treated appropriately. Neuro-physiological testing is much less frequently performed but may enhance understanding and provide additional information, often in most challenging patients. The purpose of this document to discuss pelvic floor electrodiagnostic techniques including surface and needle electromyography (EMG), nerve conduction and terminal latency studies, evoked potentials, and reflex response studies. The clinical, urodynamic, and electrophysiologic findings to be expected with neuropathy in various areas, from the cerebral cortex to the peripheral pelvic floor nerves, are also described. Because of widespread technical advances and great increase in the amount of information about human neuro-urology, concepts are continually undergoing modification and change. This chapter also concentrates on the present aspects of clinically useful knowledge, although modification of many concepts will soon be needed. EMG requires additional expertise but should be considered in the difficult clinical situation. Neurophysiology of the Lower Urinary TractIndications of Electromyography (EMG):EMG requires additional expertise but should be considered in the following types of patients: Techniques of EMG:EMG is used primarily to study striated muscle, which is easy because of sodium-mediated high-current flow. Smooth muscles, being phylogenetically earlier, depend on calcium ion exchange with low current generation. Motor unit territories within skeletal muscles are variable in size, ranging from 2 to 10 mm. To be recorded, the amplifiers and recording device must have wide-frequency response capabilities, from 30 to 10,000 Hz.
Needle EMG: inserting a needle electrode into skeletal musculature allows analysis of individual motor units and is the superior technique used by the electromyographer for studying peripheral skeletal neuromuscular disease. The needle electrodes by design may be monopolar, concentric, or single-fiber in type, varying in dimensions and in the metal used. Monopolar Needle EMG: it is made of solid stainless steel wire, 0.3 to 0.5 mm in diameter, which is insulated with Teflon except at its sharp tip. A second electrode is less painful for the subject and less expensive. Compared with concentric needles, the recorded motor unit action potentials (MUAPs) have higher amplitudes, although durations are similar. The monoplar electrode is less selective, however, which may be a disadvantage when trying to isolate single recruited MUAPs, and they have more recording artifact. Concentric Needle Electrodes (CNE): these electrodes have a hollow cannula, which serves as the reference electrode; extending down the shaft to the tip is an insulated fine wire with a beveled tip, which is the active electrode. The advantage of the concentric electrode is its more predictable surface area, which produces more reliable measurements of MUAP variables. Concentric needle study of sphincter musculature begins as soon as the needle is inserted into the muscle. Characteristic noise occurs when a muscle is penetrated (insertional activity), normally quieting soon after insertion. Observation of tonic firing of motor units in the sphincter is ideal for individual motor unit analyses. A trigger delay on the electrodiagnostic equipment is helpful because it allows a motor unit to appear repeatedly at the same point on the oscilloscope screen. The duration, amplitude, and number of turns can then be assessed. Analyses of motor units from the normal urethral sphincter reveal durations less than 6 ms, amplitudes between 0.15 and 0.5 mV and no more than 5 turns of 100 ÁV or more amplitude. More turns represent polyphasia. The number of phases in a MUAP is equal to the number of baseline zero crossings plus 1, and MUAPs having greater than four phases are considered polyphasic. Single-Fiber EMG (SFEMG): it is a selective recording technique that uses a concentric needle electrode to identify and record action potentials from individual muscle fibers. The technique is selective because of the small recording surface (25 Ám in diameter) exposed at a port at the side of the electrode 3 mm from the tip. This distance allows examination of an area approximately 350 Ám diameter. In most laboratories, high filter setting is 10,000 Hz and low setting is 350 Hz. The images are triggered and delayed using a gain setting of 100 ÁV to trigger. In other skeletal muscle work, 200 ÁV is used as the triggering potential, but in sphincter work measuring smaller muscle fibers, 100 ÁV as a trigger is now standard. The number of fibers supplied by a branch axon (hence, firing essentially simultaneously) is observed, as each fiber creates an action potential. Measurements are made visually from the oscilloscope screen. At least 20 sites within the muscle are studied, usually requiring four needle insertions, which analyze five different action potentials with each insertion. The fiber density in SFEMG recordings increases after the age of about 60 years, so that average fiber density in a normal 30-year-old is approximately 1.4, at age 65 about 1.5, and at age 75 about 1.75. Nerve Testing:
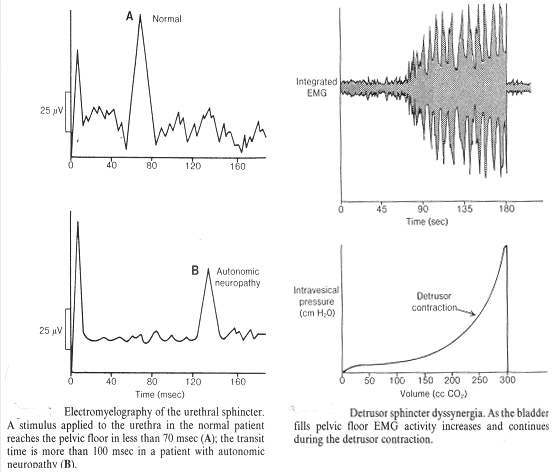
Conduction Studies: Electrical activity traveling through a nerve process can be measured by stimulating the nerve to depolarize it, thereby achieving a propagated action potential traveling away from the site of the stimulus. One may record the traveling potential directly from the nerve or when the impulse reaches a muscle, at which point the compound muscle action potential (CMAP) may be recorded. Propagation of the impulse along a nerve fiber in the same direction as occurs physiologically is called orthodromic conduction; propagation in the opposite direction is called antidromic conduction. Nerve conduction rates vary directly with the size of the nerve. Conduction velocities are also affected by temperature, with cooling slowing superficial nerve velocity by 0.7 to 2.4 m/sec for each degree centigrade. Generally, age has little significance or effect on conduction velocities until after age 60. The stimulation is accomplished by using two electrodes; a cathode and an anode. The cathode depolarizes the nerve and the anode hyperpolarizes it. Recording is accomplished with both active and reference electrodes. When studying the CAMP generated after stimulation of a nerve, the resultant latency includes the time for the nerve conduction plus the time for neuromuscular junction transmission and muscle fiber depolarization. Placement of the ground electrode on the patient is necessary, but position is not extremely critical. If convenient, the electrode is positioned between the stimulating and recording electrodes. Amplifier setting must be constant because increasing the sensitivity of the amplifier may shorten the latency value. Motor responses (CAMPs) are usually of greater amplitude and of greater amplitude and of longer duration than sensory responses recorded from the nerve itself. Pudendal Nerve Terminal Motor Latency (PNTML): The stimulating electrodes are located on the fingertip, and the recording electrodes are found at the base of the finger. The pudendal nerve is stimulated transrectally near its passage in Alcock's canal by the ischial spine, and the response is obtained from the inferior hemorrhodial division of the pudendal nerve supplying the anal sphincter. Both right and left nerves may be tested. In most of the laboratories PNTML mean is 2.2 ▒ 0.4 ms. The variables in amplitudes of the CMAP have been due to the size of the examiner's finger and the variable intraanal location in respect to the external anal sphincter. Therefore, standard positioning of the recording electrodes on paraanal skin (3 or 9 o'clock position for active electrodes and 6 o'clock for reference electrodes with patient in the lithotomy position) results in more consistency in amplitude, allowing this valuable parameter to be useful clinically. This procedure can be performed vaginally, a much more comfortable procedure for most women than intraanal stimulation. In most of the laboratories amplitude mean is 99 ▒ 44 ÁV with a range of 34 to 182 and interrater variability of 1%. Perineal Nerve Terminal Motor Latency (PeNTML): This nerve branch of the pudendal nerve supplies the area of anatomic distribution, which is even more affected by vaginal delivery than is the PNTML. Stimulating the pudendal nerve as previously described for PNTML and recording with the Foley catheter ring electrode enable one to obtain the right and left PeNTML to the urethral sphincter. Most laboratories report a mean PeNTML of 2.29 ▒ 0.3 ms. There is anatomic variability of the perineal branch of the pudendal nerve, even from side to side in the same patient. If the perineal nerve branches from the pudendal trunk distal to the point of stimulation at the ischial spine, the Foley mounted ring electrodes will record a negative-positive waveform similar to other CMAPs. However, if the urethral rhabdosphincter is supplied by a nerve supply separate from one arising from a pudendal trunk (e.g., an intrapelvic pathway from direct sacral branches), such a waveform may not be produced. Evidence of more proximal peripheral neuropathy in pelvic floor disorders has been obtained with direct spinal cord stimulation using surface electrodes and recording the latency of response in the anal and urethral sphincters. Magnetic stimulators are now being developed that may provide direct stimulation tests to evaluate more effectively the proximal pelvic floor. Somatosensory-evoked Potentials:Somatosensory-evoked potentials are the potentials recorded with surface electrodes from the central nervous system in response to stimulation of peripheral nerves. They are of low amplitude, usually less than 10 mV, and are extracted from the ongoing background activity (EEG when recording from the cortex, background noise when recording over the spinal cord) using averaging techniques. Overall, the latency of the pudendal-evoked potential is of a similar latency to the tibial evoked potential in any individual. Little diagnostic weight can be placed on the amplitude of the pudendal-evoked potential because the lower limit of normal has not been well defined. Pudendal cortical evoked responses, obtained by stimulation of the dorsal never of the penis in men, can be obtained by lateral para-clitoral stimulation in women. The two cortical recording electrodes are placed as follows: the active electrode is placed midline, 2 cm posterior to the halfway mark between the inion and the nasion, and the reference electrode may also be placed over the vertebral column at levels L1 (active electrode) and L5 (reference electrode), which correspond to the cord levels of the cauda equine. Then, using two channels, the evoked responses can be simultaneously recorded from the cortex and the lumbar spine. Such lumbar spine recording of pudendal stimuli is technically difficult in women. Similar recordings may be obtained by stimulating posterior to the medial malleolus at the posterior tibial nerve.
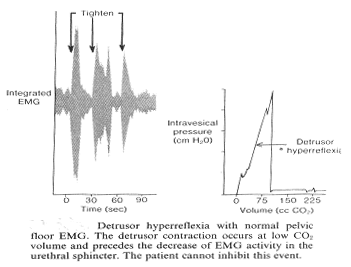
Clinical Application:The nervous system relationship to the lower urinary tract is extremely complex, and simplification, although clinically useful, will never be totally accurate. Every nervous system physiologic control over the urinary tract has positive and negative aspects, and either can dominate in a given clinical situation. Electromyography (EMG) is commonly performed with perineal surface patch electrodes (PSPE). Concentric needle electrodes (CNE) are an alternative method. Although PSPE are more comfortable, they are not as accurate in recording urethral neuromuscular activity as CNE. Other muscle activity (eg, the levator ani) affects the PSPE and is not always in unison with the urethral sphincter. PSPE are not as reliably interpretable as CNE (67% vs 89% in some studies) and do not illustrate as frequently the expected electrical quiescence. Sphincter activity is silent during voiding, and shows a slow increase during filling. There is a sharp increase in activity with coughing, Valsalva, and voluntary inhibition of a voiding episode. These increases should not be confused with detrusor sphincter dyssynergia. Quantitative EMG is being used increasingly to study urethral sphincter and pelvic floor sphincter neuromuscular activity. There is some evidence that it is superior to maximum urethral closure pressure (MUCP) in predicting the outcome of surgery for genuine stress incontinence. In some studies MUCP versus quantitative EMG activity at baseline and at bladder capacity, there was no increase in MUCP with filling, whereas quantitative EMG activity has been noted to increase from 8 ÁV to 19 ÁV, suggesting the MUCP does not adequately reflect neuromuscular urethral activity. MUCP in postmenopausal women is significantly lower than in pre-menopausal women, and the quantitative EMG activity also is lower but to a lesser degree. This supports data indicating that there is less muscle tissue and less nerve tissue in the urethra in postmenopausal women, possibly more related to age than to hormonal status. Standardization of this test may allow correlation of quantitative EMG activity with urethral neuromuscular function in genuine stress incontinence, and possible with the outcomes of incontinence surgery. One may not conclude that there is no peripheral neuropathy merely because nerve conduction velocities are normal. "Super-sensitivity" Testing: Once and organ is deprived of its efferent nerve supply, it develops super-sensitivity to its neuromuscular transmitter. This is particularly true of smooth muscle. In cases of detrusor hypoactivity, a neurogenic etiology (detrusor areflexia) may be documented by injection of bethanechol chloride (Urecholine, 5 mg subcutaneously) after baseline cystometry, followed by rapid fill cystometric studies at 5-minute intervals. A positive test is indicated by a rise in intravesical pressure more than 15 cm H2O. Bladders that demonstrate such denervation have damage to postganglionic parasympathetic nerve fibers. Reflex Response: Stimulation delivered by the Foley catheter ring electrode produces a reflex response at the external anal sphincter, also called electromyelography (myelo referring to the spinal cord). This reflex stimulus may be in urethra or may be moved into bladder to stimulate bladder. It therefore incorporates detrusor and urethral sensory afferents, conus medullaris synapses, and pudendal motor neurons. The stimulus has a pulse duration of 50 Ás, is paired with interspike interval of 5 ms, and can be increased by increments using a constant current stimulator to determine the sensory threshold. Normal urethral perception ranges from 3 to 10 mA, with a mean of 5, whereas bladder perception normally ranges 20 to 25 mA. The stimulus level is 3 to 4 times sensory threshold; most laboratories have mean of 59.0 ▒ 9.0 ms. Cauda equine or pelvic plexus injury is characterized by diminished amplitude or absence of this response.
Summary:The application of electromyography (EMG) techniques to the clinical investigation of the patient with incontinence shows that "stress incontinence" is not always due to purely anatomical reasons. Neuropathic changes in the detrusor muscle and abnormalities of the central innervation of the detrusor muscle and its reflex pathways produce incontinence by facilitating uninhibited bladder contractions. The presence of neuropathic changes in the urethra as defined by electromyographic techniques emphasizes the need to include the autonomic neuropathies associated with diabetes, multiparity and/or aging in the differential diagnosis of incontinence. The EMG promises to be of great value in the study of incontinent patient. Suggested Reading:
|
 Perineal Surface Patch Electrodes (PSPE): these are commonly used in conjunction with urodynamic studies. Two electrodes are placed -- the active placed close to the muscle under study and a remote electrode placed at a more distant site. If necessary, both recording electrodes can be placed over the active muscle. Skin surface electrodes are often used to record perineal EMG. Monopolar electrodes are placed on either side of the anal orifice. A disposable surface recording electrode, such as a silver chloride disk, can be mounted on a self-adhesive sticker. It is important to reduce the electrical resistance of the skin by washing and drying the area and applying electrode paste to ensure firm adherence between electrode and skin. When surface electrodes are used in conjunction with the cystometrogram, a gradual increase in EMG activity is usually seen as the bladder is filled.
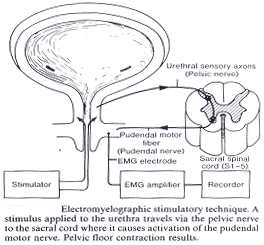
Perineal Surface Patch Electrodes (PSPE): these are commonly used in conjunction with urodynamic studies. Two electrodes are placed -- the active placed close to the muscle under study and a remote electrode placed at a more distant site. If necessary, both recording electrodes can be placed over the active muscle. Skin surface electrodes are often used to record perineal EMG. Monopolar electrodes are placed on either side of the anal orifice. A disposable surface recording electrode, such as a silver chloride disk, can be mounted on a self-adhesive sticker. It is important to reduce the electrical resistance of the skin by washing and drying the area and applying electrode paste to ensure firm adherence between electrode and skin. When surface electrodes are used in conjunction with the cystometrogram, a gradual increase in EMG activity is usually seen as the bladder is filled.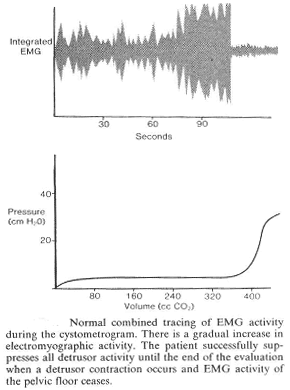 The pelvis is no different from any other area of the body and nerve testing with nerve conduction studies, reflexes and needle EMG can be performed. Interneuronal conduction occurs at nerve synapses and at neuroeffector junctions such as muscle fibers. Interneuronal communication is performed chemically by neurotransmitters, which either excites (depolarize) or inhibit (hyperpolarize) the post-synaptic membrane. Specific receptor proteins for each neurotransmitter are in the membranes on both sides of the synapse, and binding opens channels for the current flow. Many nerve cell axons have a myelin sheath, capacitating this current flow, which is interrupted at intervals called nodes of Ranvier. The amount of myelinization determines the diameter of the nerve, and the larger nerves with more myelinization have greater conduction speeds. The conduction velocity along the axon is proportional to the internodal distance. The internodal distance is decreased in neuropathy of axonal degeneration or demyelination origin. The velocity of the current flow is recorded in nerve conduction studies. In a muscle, the fibers innervated by branches emanating from the motor neuron of a single anterior horn cell are called a motor unit. The electrical activity of motor units in a muscle is recordable as motor unit action potentials (MUAPs).
The pelvis is no different from any other area of the body and nerve testing with nerve conduction studies, reflexes and needle EMG can be performed. Interneuronal conduction occurs at nerve synapses and at neuroeffector junctions such as muscle fibers. Interneuronal communication is performed chemically by neurotransmitters, which either excites (depolarize) or inhibit (hyperpolarize) the post-synaptic membrane. Specific receptor proteins for each neurotransmitter are in the membranes on both sides of the synapse, and binding opens channels for the current flow. Many nerve cell axons have a myelin sheath, capacitating this current flow, which is interrupted at intervals called nodes of Ranvier. The amount of myelinization determines the diameter of the nerve, and the larger nerves with more myelinization have greater conduction speeds. The conduction velocity along the axon is proportional to the internodal distance. The internodal distance is decreased in neuropathy of axonal degeneration or demyelination origin. The velocity of the current flow is recorded in nerve conduction studies. In a muscle, the fibers innervated by branches emanating from the motor neuron of a single anterior horn cell are called a motor unit. The electrical activity of motor units in a muscle is recordable as motor unit action potentials (MUAPs).