Urodynamic Assessment: TechniquesWHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC). An abundance of new diagnostic procedures, methodologies, and testing equipment have made it exceedingly difficult for the clinician to decide what tests are necessary to adequately evaluate lower urinary tract dysfunction in women. To understand the fundamental value of urodynamics, one should realize that the female bladder responds similarly to a variety of pathologies. Symptoms do not always reflect accurately the physiologic state of the bladder. Cystometry is an urodynamic test that measures the pressure and volume relationship of the bladder. It is used to assess detrusor activity, sensation, capacity, and compliance. Every factor has unique implications, and before any definitive conclusions can be reached, each parameter must be examined in association with symptoms and clinical findings. Despite the widespread use of cystometry, the optimal technique for performing the test is unknown. Urodynamic assessment of bladder filling and voiding function, along with urethral pressure profilometry, is typically the first study in the evaluation of the patient with complex urinary incontinence. Further studies may include static voiding cystourethrography, fluorourodynamics, ultrasonography studies of the bladder and urethra, and MRI. Further evaluation is recommended when conservative measures have failed, and invasive, potentially morbid surgical therapies are being considered. It is also indicated in patients who have new, troublesome symptoms or complications following treatment. The purpose of this document is to address the various technical aspects, controversies and techniques for performing cystometry. Cystometry (CMG) has been described as the reflex hammer of the urodynamicist. It is not only the method by which the pressure/volume relationship of the bladder is measured, but it is also an interactive process that permits examination of motor and sensory function. The International Continence Society (ICS) had defined certain terms that are used in the reporting of cystometric results. Definitions:Intravesical pressure (Pves) -- is the pressure within the bladder.
Compliance (C): is the change in volume for a change in pressure. It is calculated by dividing the volume change (∆V) by the change in detrusor pressure (∆Pdet) during that change in bladder volume (C= ∆V/ ∆Pdet). Compliance is expressed in milliliters per centimeter H2O. 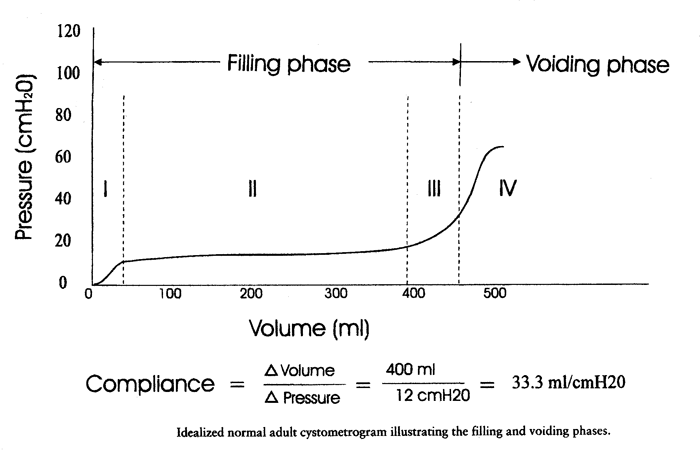 Normal adult bladder capacity is 400-550 mL. A female bladder experiences a first desire to void at a volume of approximately 150 to 250 mL, a normal desire to void at 300 to 400 mL, and a strong desire to void at 400 to 600 mL. During filling, an initial rise in true detrusor pressure between 2 and 8 cm H2O usually occurs. The average pressure rise is approximately 6 cm H2O and never exceeds 15 cm H2O. Provocation of a normal bladder by rapid filling, change of posture, coughing, or catheter movement should not normally incite any abnormal rises in detrusor pressure. Post-void Residual Urine Volume:Post-void residual (PVR) urine is defined as the volume of fluid remaining in the bladder immediately after the completion of micturition. It can be measured directly or estimated by ultrasonography. A consistently increased residual urine volume, that reflects the usual status of that patient, indicates increased outlet resistance, decreased bladder contractility, or both. Negligible residual urine suggests normal mechanical function of the lower urinary tract function of the lower urinary tract, but may also be seen with urethral obstruction if vesical pressure is sufficient to overcome increased outlet pressure. Generally, a significant residual urine volume is indicative of relative detrusor failure, with or without outlet obstruction. It is useful (and easy) to determine residual urine volume at least twice during an urodynamic assessment, because anxiety or discomfort in addition to true pathology may be responsible for an incomplete voiding event. Measurement of post-void residual (PVR) urine volume is taking on more clinical significance as the use of bladder ultrasound for the estimation of bladder volume has become widely commercially available. By comparing the ultrasound measurements with the catheterization urine volume, the sensitivity and specificity of ultrasound detecting the presence of residual volumes of >100 mL were excellent. Even though the test-retest reliability is poor, a series of post-void residual urine measurements, when annotated by the clinical circumstances, provides useful information. It is our practice to use catheter to check residual urine volume at time of urodynamic testing when catheterization is required and to use ultrasound to measure residual volume in the office setting to avoid catheterization. Important points in the interpretation of PVR include:
A "normal" postvoid residual (PVR) volume is generally defined as 200 mL or less. This value is based on consensus recommendations from the United States Department of Health and Human Services Agency for Health Care Policy and Research (AHCPR), which were published in 1992. According to these recommendations, a PVR volume less than 50 mL is considered indicative of adequate bladder emptying, and a PVR volume greater than 200 mL is considered indicative of inadequate emptying. There is minimum evidenced-based data on which these values are based. Additionally, there are no recommendations regarding the significance of PVR volumes between 50 and 200 mL. Filling media:The commonly used infusants for cystometry include water, physiologic saline, carbon dioxide, and radiographic contrast material. Air should not be used because of the rare possibility of fatal air embolus. In our opinion, liquid is vastly superior to gas. Carbon dioxide was introduced by Merrill et al in 1971 has become popular in North America. It is particularly suitable for office studies because it is clean and quick, and can be instilled at rates of up to 300 mL/min. Nevertheless, the following reservations about the use of gas during cystometry exist. First, it further decreases the physiologic nature of the test. Second, if gas is used, the bladder volume cannot be assessed because CO2 is compressible. Third, CO2 dissolves in urine to form carbonic acid, which irritates and reduces functional bladder capacity. Fourth, abdominal pressure is not usually measured during CO2 cystometry, making interpretation more difficult. Finally, when CO2 is used for filling cystometry, it is impossible to perform a stress test or voiding studies. Water or physiologic saline is the most commonly used filling medium unless radiologic screening is also being performed, in which case contrast medium is used. The cystometric findings are not affected by the choice of liquid medium. Most laboratories use fluid at room temperature, although some investigators believe that the instillation of warm or cold fluid may provoke abnormal bladder activity. The instillation of ice water (Bor's test) is occasionally used as a test for neurologic disorders. The International Continence Society (ICS) attempted to standardize filling rates by describing three ranges: slow fill is less than 10 mL/min, medium fill is 10 to 100 mL/min, and rapid or fast fill is greater than 100 mL/min. Patients with normal lower urinary tract function can tolerate most fast-fill rates. The effect of filling rate on an unstable bladder is still poorly understood, but fast-fill methods may be more effective in provoking detrusor over-activity. For this reason, medium- or fast-fill techniques are more widely used. In patients with neurologic abnormalities, slow fill is essential to reduce artifactual bladder activity. Position of patient and provoking maneuvers:Cystometry should mimic everyday stresses on the bladder as much as possible. Thus, it is preferable to perform the test with the patient in the sitting or standing position. During cystometry, the bladder should be provoked by a series of tests that usually include coughing, heel bouncing, walking in place, and listening to running water. These maneuvers may provoke uninhibited detrusor contractions or induce stress incontinence. Techniques:The patient presents with a symptomatically full bladder. She voids spontaneously in an uroflow chair. A postvoid residual urine volume is obtained via a transurethral catheter. With the catheter in place, approximately 50 mL of sterile, room-temperature saline or water is placed into the bladder to facilitate placement of the micro-transducer catheters and to decrease the amount of initial artifact secondary to the bladder wall collapsing around the micro-tip. The micro-transducer catheters are connected to the appropriate cables and to the tubing from the water pump. The machine is calibrated with the catheters in water and all channels are set at zero. A small amount of water is flushed through the tubing to remove any air. With the patient in the supine position on an urodynamic chair, the abdominal catheter is place into the vagina and taped to the inside of the leg. If the patient has severe vaginal prolapse or has undergone previous vaginal surgery resulting in a narrowed vagina, the catheter is placed into the rectum. A dual micro-transducer catheter with a filling port is then placed into the bladder. The patient is moved to a sitting position and the catheter secured to a mechanical puller (if urethral pressure studies are anticipated) or to the inside of the leg, so that the proximal transducer is near the mid-urethra (area of maximum urethral closure pressure). After the catheters are appropriately placed, the subtraction is checked by asking the patient to cough. Cough-induced pressure spikes should be seen on the Pves, Pabd, and Pure channels, but not on the true detrusor pressure channel. If there is an inappropriate deflection on Pdet channels, but not on the true detrusor pressure channel. If there is an inappropriate deflection on Pdet, it is usually secondary to inaccurate placement of the vaginal (or rectal) catheter. If repositioning the catheter does not correct the problem, all connections and calibration techniques should be rechecked. Bladder filling is begun. First sensation, initial urge to void, and maximum capacity are recorded. Throughout the filling portion of the examination, the patient is asked to perform provocative activities, such as coughing and straining. The external urethral meatus is constantly observed for any involuntary urine loss. Leak point pressures can be obtained at various bladder volumes. Any abnormal rise in true detrusor pressure is noted. If the patient's symptoms are reproduced during filling, then the test can be completed in the sitting position. If they are not, the patient should be asked to stand and perform provocative maneuvers in an attempt to reproduce her symptoms. At the completion of filling, urethral pressure and flow studies can be performed, if indicated. An example of filling and voiding cystometry with no abnormalities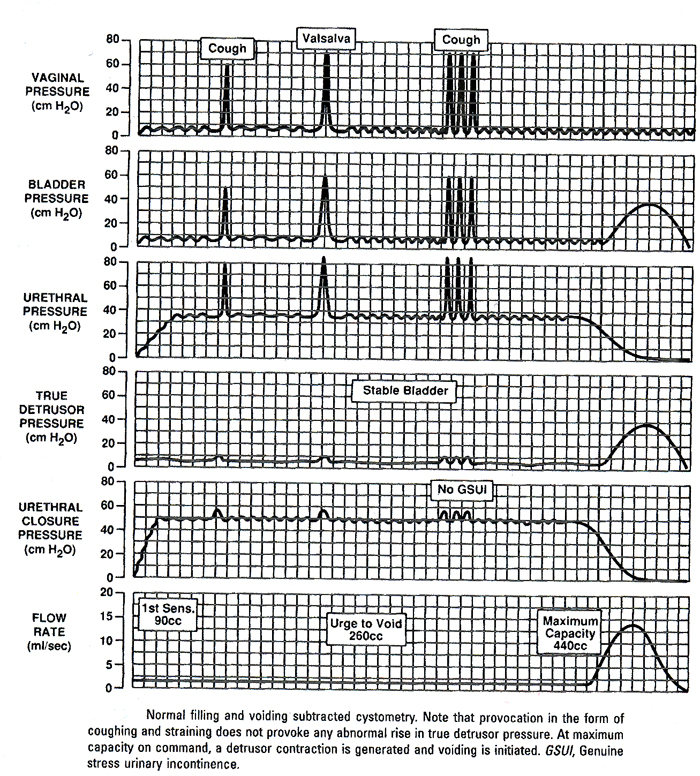 Video-Urodynamic Testing:Video-urodynamic studies of the lower urinary tract represent a combination of video-cystourethrography and standard urodynamic techniques. Video-urodynamic requires equipment of cystometry, plus an image intensifier and a videotape recorder. In addition, various interface modules are necessary, depending on the exact design of the system. A television camera positioned above the recorder with a mixing device projects the recording channels on a television monitor alongside the radiographic image of the bladder. Radio-opaque filling medium is used for video-urodynamic studies. With all the urodynamic studies, every effort must be made to limit the inhibitory effect of the additional machinery and personnel imposed on the patient. Potential advantages of video-urodynamic studies include the consolidation of multiple evaluation modalities into one examination, thereby providing information about lower urinary tract anatomy and function under various provocative environments. Descent of the bladder neck, milk-back of urine from the urethra to the bladder, and bladder neck funneling all may be visualized during simultaneous recording and imaging of bladder, urethral, and abdominal pressures. Asymptomatic abnormalities such as urethral or bladder diverticula also may be noted. The major disadvantages of video-urodynamic testing are the radiation exposure, cost, and technical expertise and support necessary for its use. In our judgment, when flow or post-void residual (PVR) urine is abnormal, simple cystometry is of marginal use. Video-urodynamics is most important in the assessment of complex cases and in patients for whom it is important to evaluate detrusor function. Eyeball Urodynamics:A simple "eyeball Cystometrogram (CMG)" can be performed at the bedside or in the office. The patient is placed in the supine position and catheterized with an 18 Fr catheter. Post-void residual urine is measured. The plunger is removed from a 50-60 mL catheter tip syringe and the barrel of the syringe is connected directly to the end of the catheter. Water or saline is infused into the bladder by pouring into the open end of the syringe. The height of the barrel is raised or lowered until there is steady flow. The patient is instructed to neither try to void nor to inhibit micturition, but rather to report their sensations to the clinician. If the rate of infusion begins to slow down or if the fluid begins to back up, the barrel of the syringe is raised or lowered until flow just stops. The height of the meniscus, above the symphysis, is a measure of vesical pressure. If the pressure does rise, it may be due to and involuntary detrusor contraction, to an increase in abdominal pressure or to low bladder compliance. Increases in intra-abdominal pressure are usually detectable by visual observation or palpation of the abdomen, but low bladder compliance may be difficult to distinguish from an involuntary detrusor contraction. An involuntary detrusor contraction is characterized by a sudden increase in pressure which is not volitional. In most neurologically normal patients the involuntary detrusor contraction is perceived by the patient as an urge to void, but some patient may be completely unaware of it. If involuntary detrusor contractions are suspected, but not demonstrated, the examination is repeated in the upright position. The patient is asked to cough or strain and the pressure response observed to see if this stimulates an involuntary detrusor contraction, which will be apparent as a sustained increase in pressure that persists long after the increase in abdominal pressure has abated. Endourodynamics:Endourodynamics describes the technique of performing CMG through the working port of a flexible cystoscopy. The first combined flexible cystoscopy and urodynamic evaluation was reported in 1986 by Loughlin and associates. Douenias et al have confirmed the effectiveness and accuracy of endourodynamics, using flexible cystoscopy to monitor bladder neck function during detrusor contraction. We evaluate large numbers of spinal cord-injured patients with both cystoscopic and urodynamic evaluation. In many cases, urologic signs and symptoms mandate both tests. In other cases, where initially only cystometry is anticipated, difficulty inserting an urodynamic catheter prompts cystoscopic examination. This not only defines the nature of the difficulty but also permits urodynamic study through the endoscope. Radiographic contrast solution is used as the CMG infusants when video urodynamics is anticipated. Periodic observation with both direct vision and fluoroscopy assessed endoscope position, bladder configuration, vesicoureteral reflux, and urinary incontinence. Voiding pressure is determined when detrusor contraction caused by micturition around the flexible cystoscope. Few patients experience difficulty with this. If the patient is unable to urinate with endoscope in position, its removal permits spontaneous voiding under fluoroscopic monitoring of the bladder neck, prostatic urethra, and the external sphincter. Ambulatory Urodynamics (AUDS):The largest deficiency of currently available urodynamic techniques is that laboratory observations may not always represent accurately physiologic behavior of the bladder and urethra. At times, the urodynamicist cannot reproduce the patient's symptoms in the laboratory setting. Several companies have recently developed commercially available AUDS systems. This equipment uses indwelling catheter-mounted transducers that are connected to a microcomputer worn over the patient's shoulder. This allows freedom of movement to the extent that the patient can reproduce the activities that incite lower urinary tract dysfunction. In principle these systems are the same as those used for conventional urodynamics, and the same basic methodology applies. AUDS should be considered when conventional urodynamics fail to provide a pathophysiologic explanation for the patient's symptoms. The most common example of this is in a patient who complaints of incontinence that cannot be objectively demonstrated and has failed non-surgical modes of therapy. Before more invasive therapy such as surgery is considered, AUDS could be used to objectively demonstrate the incontinence. AUDS systems are also helpful in determining whether detrusor instability or urethral incompetence is the main cause of incontinence in women for whom surgery is being contemplated. To obtain the measured data, the monitor is connected to a personal computer by means of an interface unit. With the help of a software package, the data from the monitor memory are transmitted to the personal computer. The patient and diary data are entered via the keyboard. Then, the curves are analyzed, which means that diary data and events are correlated with the measured curves. A clinical report is generated with a summary of the drinking and micturition behavior, urine loss, and analyzed detrusor and sphincter activity. Because the information one is gathering about detrusor function and voiding and drinking behavior is so extensive, and detrusor activity during the filling phase is also found in healthy asymptomatic subjects, an interpretation tool had to be developed. Based on our overall statistical analysis of patients and healthy volunteers, the detrusor activity index (DAI) was established. This index is based on model analysis regression techniques. The outcome is a mathematical rule using variables from ambulatory monitoring and diary and explains the patient's symptoms and establishes an urodynamic diagnosis. The DAI has been validated by several drug trials showing quantitative differences in efficacy, which correlated well with the clinical experiences of different urologists. We believe that ambulatory urodynamics (AUDS) will become the "gold standard" for urodynamics in the near future if several additions to the technique can be realized. Among these additions, we will need at least: integrated uroflow recording, quantitative urine loss measurement, and automatic quantitative analysis of all curves, including predictable quality of measurement interpretation. AUDS has some drawbacks. During the investigation there is no control on the validity of the measured signals. For this reason it is important that before the patient is sent away with the monitor, the catheter position must be checked and the patient must be adequately instructed. Ultrasound Urodynamics:Ultrasound is increasingly being used as an alternative imaging technique thanks to the urinary bladder, which when full serves as an acoustic window to view the urine bladder and adjacent pelvic organs. Ultrasound has many inherent advantages. Catheterization is rarely needed. Ultrasound also obviates the need for irradiation, permitting prolonged periods of visualization, and safety in repeating the examinations. It is also non-invasive and portable or mobile, without any recorded complications. Ultrasound techniques, however, require special skill, know-how, and familiarity with different kinds of probes, which can be expensive and fragile. To date, transabdominal, transrectal, transvaginal, and perineal ultrasound all have been used to visualize the lower urinary tract. Static ultrasound can be used for assessing bladder volume and filling or bladder morphology including tumors. Transurethral intravesical ultrasound with a specialized probe has also been introduced as a method of assessing the bladder wall depth of cancer invasion. Dynamic ultrasound serves as an adjunct in determining the relationships between the bladder, urethrovesical junction, urethra and pubic bones, and assessing the position and shape of the bladder base when evaluating stress urinary incontinence in conjunction with conventional urodynamics. In the near future, ultrasound will become a more popular technology not only for residual urine measurements, but also for evaluations of stress incontinence and pelvic structures. Summary:Cystometry is the most important and most commonly performed urodynamic test. Although it is clinically useful, its limitations should be recognized. Gynecologists should become familiar with cystometry so that they can perform and interpret basic office tests and work with urogynecologic or urologic consultants when more sophisticated tests are indicated. Patient cooperation is imperative in obtaining adequate cystometric studies. Moving, crying, extraneous muscular activity and failure of the patient to follow directions diminish the value of the study. Recordings of abnormal movement, or coughing should be labeled on the tracing. The operator should note all such visible artifacts so that these pressure spikes will not be interpreted as bladder contractions. After a detailed explanation to the patient, the examination is begun by passing a catheter into the bladder, measuring residual urine, and filling the bladder. Close verbal contact is maintained between patient and examiner as predefined motor and sensory landmarks are observed and annotated. Suggested Reading:
|