Gestational Trophoblastic Disease: A Comprehensive ReviewWHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).Gestational Trophoblastic disease comprises a spectrum of interrelated conditions originating from the placenta. The incidence of various forms of gestational trophoblastic disease in the United States is approximately 1 in 600 therapeutic abortions and 1 in 1,500 pregnancies. Approximately 20% of patients will develop malignant sequelae requiring administration of chemotherapy after evacuation of hydatidiform moles. Most patients with post-molar gestational trophoblastic disease will have non-metastatic molar proliferation or invasive moles, but gestational choriocarcinomas and metastatic disease can develop in this setting. The purpose of this document is to address current evidence regarding the diagnosis, staging, and management of gestational trophoblastic disease. Other terms often used to refer to these conditions include gestational trophoblastic neoplasia and gestational trophoblastic tumor. At present, with sensitive quantitative assays for beta-hCG and current approaches to chemotherapy, most women with malignant gestational trophoblastic disease can be cured and their reproductive function preserved. Histologically distinct disease entities encompassed by this general terminology include complete and partial hydatidiform moles, invasive moles, gestational choriocarcinomas, and placental site trophoblastic tumors. Gestational choriocarcinomas occurs in approximately 1 in 20,000 - 40,000 pregnancies; approximately 50% after term pregnancies, 25% after molar pregnancies, and the remainder after other gestational events (1).Hydatidiform Mole Classification: Partial and complete hydatidiform moles are distinct disease processes with characteristic cytogenetic, histologic, and clinical features. The management of patients with complete and partial moles is similar. Its distinct features are:Partial Mole: Its karyotype most commonly is 69, XXX or 69, XXY. Fetus is often present and amnion, fetal red blood cells are usually present. Focal variable villous edema and focal, slight to moderate trophoblastic proliferation are the characteristic of partial mole. Clinical presentation may be of missed abortion, uterine size may be smaller for gestational age, theca-lutein cysts and medical complications are rare. Post-molar malignant sequelae are <5%. Photographs from Rosai and Ackerman's Surgical Pathology (Mosby an affiliate of Elsevier Limited Publisher) 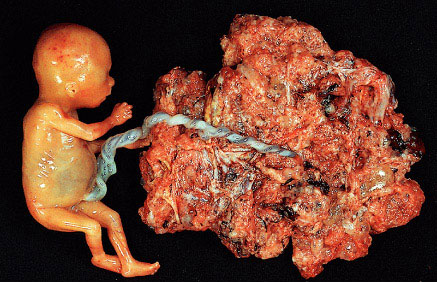
Partial mole with attached fetus. The diagnosis was confirmed by biopsy and flow cytometry. The fetus showed no abnormality and was connected to the mole by a normal umbilical cord. (Courtesy of Dr. Pedro J. Grases Galofré)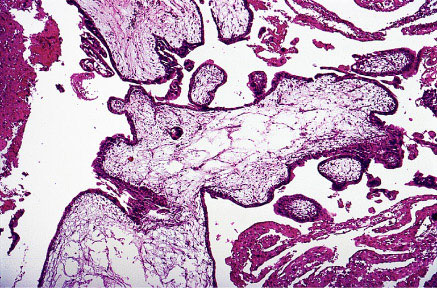
Partial mole showing scalloping of villi and isolated trophoblastic cells embedded in the stroma.Complete Mole: Its karyotype most commonly is 46, XX or 46, XY. Fetus and amnion, fetal red blood cells are usually absent. Villous edema and trophoblastic proliferation is diffuse (slight to severe). Clinical presentation usually is uterine size 50% larger for gestational age, incidence of theca-lutein cysts is about 15-25%. Post-molar malignant sequelae are about 6-32%. 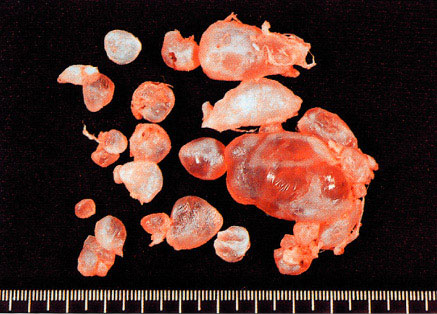
Complete mole. All villi are markedly swollen. (Courtesy of Dr. Pedro J. Grases Galofré)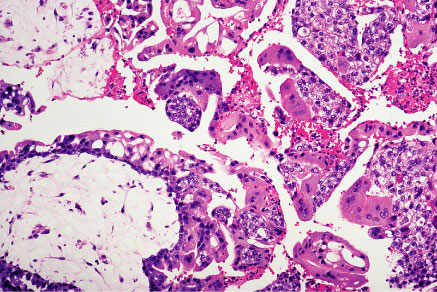
Complete mole showing large villi with stromal edema and marked trophoblastic proliferation.Diagnosis: Hydatidiform moles usually are diagnosed during the first trimester of pregnancy. The most common symptom is abnormal bleeding. Other signs and symptoms include uterine enlargement greater than expected for gestational age, absent fetal heart tones, cystic enlargement of the ovaries, hyperemesis gravidarum, and an abnormally high level of hCG for gestational age. Presence of these features in the first trimester should alert the clinician to the possibility of a molar gestation. Pregnancy-induced hypertension in the first half of pregnancy, although uncommon, is suggestive of hydatidiform mole. Ultrasound findings of diffuse mixed echogenic pattern replacing placenta, produced by villi and intrauterine blood clots are the diagnostic features of molar pregnancy.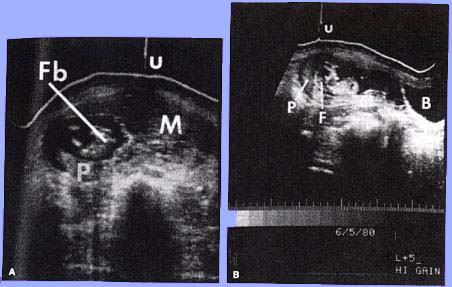
Coexistent mole and fetus. (A) Transverse scan illustrating fetal body (Fb), placenta (P), hydatidiform mole(M), and umbilicus(u), (B)Longitudinal scan 5 cm to the right of midline. The fetus (F) is present within a gestational sac. P, placenta; B, maternal bladder; u, umbilicus. | 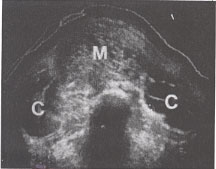
Transverse scan of a classic mole(m) with bilateral theca-lutein cysts (c). |
For patients in whom hydatidiform moles are suspected before evacuation, the following tests are recommended: - Complete blood count with platelet determination
- Clotting function studies
- Renal and liver function studies
- Blood type with antibody screen
- Determination of hCG level
- Pre-evacuation chest X-ray
To manage potential complications of molar evacuation in a woman with a large uterus, consideration should be given to performing the evacuation in a facility with an intensive care unit, a blood bank, and anesthesia services. For most patients the preferred method of evacuation is suction D&C. Evacuation usually is performed with the patient under general anesthesia, but local or regional anesthesia may be used for a cooperative patient who has a small uterus. In some cases, ultrasound guidance may facilitate complete evacuation of the uterus. Intravenous oxytocin is administered after the cervix dilated and is continued for several hours postoperatively. Rh-negative patients should be treated with anti-D immune globulin after the evacuation even though fetal red blood cells should not be present in a complete mole. Pulmonary complications are frequently observed around the time of molar evacuation among patients with marked enlarged uterus. Respiratory distress syndrome can be caused by high-output congestive heart failure caused by anemia or hyperthyroidism, preeclampsia or iatrogenic fluid overload. Hysterectomy with preservation of the adnexa is an alternative to suction D&C for molar evacuation in selected patients who do not wish to preserve childbearing. Hysterectomy reduces the risk of malignant post-molar sequelae when compared with evacuation by D&C. However, the risk of post-molar gestational trophoblastic disease after hysterectomy remains approximately 3-5%, and these patients should be monitored post-operatively with serial hCG levels. Management After Evacuation of Hydatidiform Mole: After molar evacuation, it is important to monitor all patients carefully to diagnose and treat malignant sequelae promptly. Oral contraceptives do not increase the incidence of post-molar gestational trophoblastic disease or alter the pattern of regression of hCG values (2). After completion of documented remission for 6-12 months, women who desire pregnancy may discontinue contraception, and hCG monitoring may be discontinued. Patients with prior partial or complete moles have a 10-fold increased risk (1-2% incidence) of a second hydatidiform mole in a subsequent pregnancy. Therefore, all future pregnancies should be evaluated by early obstetric ultrasound. A variety of hCG criteria have been used to diagnose post-molar gestational trophoblastic disease. Recently, the International Federation of Gynecologists and Obstetricians (FIGO) standardized hCG criteria for the diagnosis of post-molar gestational trophoblastic disease (3). The following criteria are proposed by FIGO:- An hCG level plateau of 4 values plus or minus 10% recorded over a 3-week duration (days 1,7,14 and 21).
- An hCG level increase of more than 10% of 3 values recorded over a 2-week duration (days 1,7, and 14).
- Persistence of detectable hCG for more than 6 months after molar evacuation.
A new intrauterine pregnancy should be ruled out on the basis of hCG levels and ultrasonography, especially when there has been a long delay in follow-up of serial hCG levels and non-compliance with contraception. The diagnosis of malignant sequelae as indicated by the need for chemotherapy include the plateau or increase of hCG levels after evacuation of hydatidiform moles as mentioned previously, the histologic diagnosis of choriocarcinomas or invasive mole on the basis of findings from uterine curettage, or identification of clinical or radiologic evidence of metastases. As long as hCG values are decreasing after molar evacuation, there is no role for chemotherapy. However, if hCG levels increase or plateau over several weeks, immediate evaluation and treatment for malignant post-molar gestational trophoblastic disease are indicated. Role of Prophylactic Chemotherapy: There are anecdotal cases of fatalities caused by prophylactic chemotherapy and prophylactic chemotherapy does not eliminate the need for post-evacuation follow up. In compliant patients, the low morbidity and mortality achieved by monitoring patients with serial hCG determinations and instituting chemotherapy only in patients with post-molar gestational trophoblastic disease outweighs the potential risk and small benefit of routine prophylactic chemotherapy. Two randomized studies have evaluated prophylactic chemotherapy after molar evacuation (4). In one study, a single course of methotrexate and folinic acid reduced the incidence of post-molar trophoblastic disease from 47.4% to 14.3% ( p<.05) in patients with high-risk moles (as defined by hCG levels greater than 100,000 mIU/mL, uterine size greater than gestational age, and ovarian size greater than 6 cm), but the incidence was not reduced in patients with low-risk moles. Patients who received prophylactic chemotherapy but developed post-molar trophoblastic disease required more chemotherapy than those who had not been exposed to prophylactic chemotherapy. In second study, a single course of prophylactic dactinomycin was given to patients after evacuation of high-risk moles. Post-molar trophoblastic disease occurred in 50% of the control group, compared with 13.8% of the treatment group. In both studies there were no deaths in the treatment or control groups caused by gestational trophoblastic disease or treatment toxicity. Hydatidiform Mole and Co-existent Fetus: Co-existence of a fetus with molar changes of the placenta is relatively rare, occurring in 1 in 22,000 -100,000 pregnancies. A variety of criteria have been used to evaluate these pregnancies. Compared with singleton hydatidiform moles, twin pregnancies with a fetus and a mole carry an increased risk for post-molar gestational trophoblastic disease, with higher proportion of patients having metastatic disease and requiring multi-agent chemotherapy. Among patients with co-existent moles and fetuses who continue pregnancy, a subset develops early complications leading to termination of the pregnancy before fetal viability, with a markedly increased risk of post-molar gestational trophoblastic disease, when compared with patients whose pregnancies continue into the third trimester. Major congenital abnormalities have not been reported in surviving infants. For patients with co-existing hydatidiform moles and fetuses suspected on the basis of ultrasound findings, there are no clear guidelines for management. If the continuation of pregnancy is desired, fetal karyotype should be obtained, a chest X-ray performed to screen for metastases, and serial serum hCG values monitored. These patients are at an increased risk for medical complications of pregnancy requiring evacuation, including bleeding, preterm labor, and pregnancy-induced hypertension. They should be counseled about these risks and the increased risk of post-molar trophoblastic disease after evacuation or delivery. If the fetal karyotype is normal, major fetal malformations are excluded by ultrasound examination, and there is no evidence of metastatic disease, it is reasonable to allow the pregnancy to continue unless pregnancy-related complications force delivery. After delivery, the placenta should be histologically evaluated and the patient followed closely with serial hCG values, similar to management of a woman with a singleton hydatidiform mole. "Phantom hCG" or False-Positive hCG values: Rarely, patients have persistently elevated hCG levels but are subsequently found to have a false-positive hCG assay result, sometimes after receiving chemotherapy or surgery for presumed malignant gestational trophoblastic disease. Most patients with false-positive hCG values have low-level hCG elevations, but occasionally values higher than 300 mIU/mL have been recorded. False-positive hCG values result from interference with hCG immunometric sandwich assays, most often caused by non-specific heterophilic antibodies in the patient's sera (5). Many of these patients have an undefined previous pregnancy event and do not have radiographic evidence of metastatic disease. It is important to exclude the possibility of false-positive hCG values before subjecting these patients to hysterectomy or chemotherapy for gestational trophoblastic disease. Malignant Gestational Trophoblastic Disease: Clinical Presentation and Diagnosis:The clinical presentation of malignant gestational trophoblastic disease is more important in determining treatment and outcome than the precise histologic diagnosis. It comprises of:- Non-invasive trophoblastic proliferation
- Invasive moles
- Gestational choriocarcinomas
The rarest form of malignant gestational trophoblastic disease, placental site trophoblastic tumor, can follow any pregnancy. The term invasive mole is used to describe disease confined to the uterus and is characterized by the presence of edematous chorionic villi with trophoblastic proliferation that invade directly into the myometrium. Dilation and curettage (D&C) should be avoided to prevent morbidity and mortality caused by uterine perforation. Gestational choriocarcinomas tend to develop early systemic metastasis (vagina, lung, liver, and brain are the most common sites), and chemotherapy should be initiated in a timely manner to avoid bleeding complications at metastatic sites. Placental site trophoblastic tumors are relatively rare and are characterized by absence of villi with proliferation of intermediate trophoblast cells. Relatively lower levels of hCG are secreted by these tumors (6). Surgery assumes a critical role in the management of placental site trophoblastic tumors and most patients have disease confined to the uterus and are cured by hysterectomy. Photographs from Rosai and Ackerman's Surgical Pathology (Mosby an affiliate of Elsevier Limited Publisher) 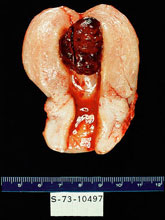 | 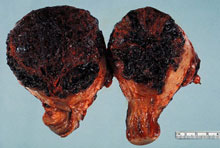
Uterine choriocarcinoma showing typical highly hemorrhagic appearance. | 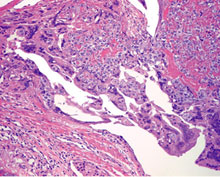
Intimate admixture of syncytiotrophoblast and cytotrophoblast in choriocarcinoma. |
Post-molar gestational trophoblastic disease is most frequently diagnosed on the basis of increasing or plateauing hCG values. Women with malignant gestational trophoblastic following non-molar pregnancies may have subtle signs and symptoms of disease, which make the diagnosis difficult. Abnormal bleeding for more than 6 weeks following any pregnancy should be evaluated with hCG testing to exclude a new pregnancy or gestational trophoblastic disease. Staging and Classification of Malignant Trophoblastic Disease: Three systems have been used to categorize patients with malignant gestational trophoblastic disease:- The World Health Organization (WHO) prognostic index score
- The Clinical Classification system developed from early experience with chemotherapy for patients treated at the National Institutes of Health (NIH)
- The FIGO staging system, which was revised in 2000.
The 2000 FIGO modification of the WHO prognostic index score eliminated the determination of patient and consort blood types because these are not uniformly available and consolidated the risk categories into low-risk (total score less than 7) and high-risk (total score of 7 or higher) categories. The new FIGO risk index also standardized the radiologic studies to be used for determining the number and size of metastases. The Clinical Classification led by NIH is frequently used in the United States. This system segregates patients with non-metastatic disease because virtually all patients with non-metastatic disease can be cured using initial single agent chemotherapy, regardless of low-risk. Patients with metastatic disease are further sub-divided depending on the presence or absence of factors that correlate with response to initial single-agent chemotherapy. Patients who have any high-risk clinical factor are classified as having poor-prognosis disease. These patients are not only at an increased risk of failure of single-agent chemotherapy but also have an increased risk of death if treated with single-agent therapy followed multi-agent regimens when compared with patients receiving initial multi-agent regimens (7). Virtually all deaths from malignant gestational trophoblastic disease occur among women who fall into the poor-prognosis metastatic disease category, and these patients should be considered to have high-risk disease. General considerations for the evaluation of malignant gestational trophoblastic disease: Once the diagnosis of malignant gestational trophoblastic disease is suspected or established, immediate evaluation for metastases and risk factors is mandatory. Along with history and physical examinations, the following laboratory studies should be performed: complete blood count with platelet determinations, clotting function studies, renal and liver function studies, blood type and antibody screen, and determination of baseline (pre-therapy) hCG level. X-ray chest or computerized tomography (CT) scan of the chest, pelvic ultrasonography, brain magnetic resonance imaging or CT scan, and abdomino-pelvic CT with contrast or magnetic resonance imaging scans are recommended to evaluate the extent of metastases. Venous metastasis of malignant gestational trophoblastic disease results in pulmonary or occasional vaginal lesions. Arterial metastasis usually occurs only after pulmonary metastases have been established; therefore, the minimum evaluation of a patient with post-molar gestational trophoblastic disease is a chest X-ray. If lung lesions are detected, further imaging of the abdomen and brain should be performed to identify possible liver or brain metastasis. Treatment of non-metastatic gestational trophoblastic disease: Essentially all patients with this condition can be cured, usually without hysterectomy. Weekly intramuscular methotrexate at a dose of 30-50 mg/m2 is the preferred choice of treatment as reported by Gynecologic Oncology Group (8). Chemotherapy is continued until hCG values have reached normal levels; an additional course is administered after the first normal hCG value has been recorded. Hematologic indices should be monitored carefully during chemotherapy, but significant hemotologic toxicity is infrequent among patients treated with weekly methotrexate. Patients should have normal renal and liver functions before each treatment because methotrexate is excreted entirely by the kidney and can produce hepatic toxicity. Dactinomycin can also be used as a single agent therapy with good results. Early hysterectomy can shorten the duration and the amount of chemotherapy required to produce remission. Therefore, each patient's desire for future fertility should be evaluated at the onset of treatment. Many experts prefer to perform hysterectomy during the first cycle of chemotherapy and continue administration of chemotherapy for 2 cycles after a negative hCG measurement has been obtained. Chemotherapy after hysterectomy is needed until hCG values become normal. Patient's whose hCG levels reach a plateau or increase during therapy should be switched to an alternative single-agent regimen. If metastases appear or alternative single-agent chemotherapy fails, the patient should be treated with multi-agent regimens. Hysterectomy should be considered in patients who are refractory to chemotherapy and remains confined to uterus. The overall cure rate for patients with non-metastatic disease is nearly 100%.Treatment of low-risk metastatic gestational trophoblastic disease: These patients can be treated successfully with initial single-agent regimens. Most often, this consists of 5 day treatment using methotrexate or intravenous dactinomycin recycled at 14-day intervals. Approximately 40% of these patients will require alternative therapy to achieve remission. Hysterectomy in conjunction with chemotherapy also may decrease the amount of chemotherapy required to achieve remission in these patients. 1-2 cycles of chemotherapy should be given after the first normal hCG level. Recurrence rate are less than 5% among patients successfully treated for low-risk metastatic disease. Treatment of high-risk metastatic gestational trophoblastic disease: Patients with 1 or more of the Clinical Classification system risk factors or a FIGO risk score of 7 or higher have high-risk disease. They require multi-agent chemotherapy with additional surgery or radiation often incorporated into treatment. Survival rates reported by trophoblastic disease centers have been reported as high as 84%. Aggressive treatment with multi-agent chemotherapy is an important component for management of these patients. Triple therapy with methotrexate, dactinomycin and either chlorambucil or cyclophosphamide was the standard regimen for many years in the United States. More recent regimens have incorporated etoposide with or without cisplatin into combination therapy with higher rates of success with an increased risk for leukemia in survivors (9). Management of cerebral metastases is controversial. Radiation therapy has been used concurrently with chemotherapy in an attempt to limit acute hemorrhagic complications from these metastases. Brain irradiation combined with systemic chemotherapy for these metastases is successful in controlling brain metastases with cure rats up to 75% in patients. The best treatment for liver or other high-risk sites of metastases has not been established. Even with intense chemotherapy, additional surgery may be necessary to control hemorrhage from metastases, remove chemo-resistant disease, or treat other complications to stabilize high-risk patients during therapy. Chemotherapy is continued until hCG values have normalized, followed by at least 2 -3 courses of maintenance chemotherapy in the hope of eradicating all viable tumors. About 13% of high-risk disease patients develop recurrence after achieving an initial remission. Surveillance following completion of chemotherapy: After remission patients should undergo serial determinations of hCG levels at 2 week intervals for the first 3 months of remission and then at 1 month intervals until monitoring has shown 1 year of normal hCG levels. The risk of recurrence after 1 year of remission is less than 1%, but late recurrences have been observed rarely. Patients should be counseled to use a reliable form of hormonal contraception during the first year of remission. Because of the 1-2% risk for second mole in subsequent pregnancies, early ultrasound examination is recommended for all future pregnancies. There does not appear to be increase in the risk of congenital malformations or other complications related to pregnancy. Prognostic Factors and FIGO Staging System:Currently the FIGO staging system is the standard classification and used for reporting results. Patients are assigned both an anatomic stage and a risk score. In FIGO stage I, disease is confined to the uterus; in stage II, disease extends outside of the uterus but is limited to the genital structures; in stage III, pulmonary involvement is apparent by chest X-ray; and in stage IV, other systemic metastases have occurred. Thus a woman with stage IV-14 disease would have disseminated metastases and a high risk score, whereas stage I-3 would indicate disease limited to the uterus with few risk factors. The total risk score for a patient is obtained by adding the individual scores for each prognostic factor. A total score of 0-6 is low risk, whereas a score of 7 and higher is high risk (10).Prognostic factors with score of 0 points for each item are: age less than 39 years; hydatidiform mole in previous pregnancy; less than 4 months between pregnancies; less than 1,000 pretreatment hCG (milli-international units/mL); largest tumor including uterus less than 3 cm; no metastases.Prognostic factors with score of 1 point for each item are: age older than 39 years; abortion in previous pregnancy; 4-6 months between pregnancies; 1,000 to 10,000 pretreatment hCG (milli-international units/mL); largest tumor including uterus is 3-4 cm; metastases located in spleen or kidney; 1-4 metastases.Prognostic factors with score of 2 points for each item are: previous term pregnancy; 6-12 months between pregnancies; greater than 10,000 to 100,000 pretreatment hCG (milli-international units/mL); largest tumor including uterus is 5 cm or larger; metastases located in gastrointestinal tract; 4-8 metastases; previous failure of single-drug chemotherapy. Prognostic factors with score of 4 points for each item are: more than 12 months between pregnancies; greater than 100,000 pretreatment hCG (milli-international units/mL); metastases located in brain or liver; more than 8 metastases; previous failure of chemotherapy consisting of two or more drugs. Summary: Abnormal bleeding for more than 6 weeks following any pregnancy should be evaluated with hCG testing to exclude a new pregnancy or gestational trophoblastic disease. In patients with molar pregnancy, the preferred method of evacuation is suction D&C. After molar evacuation, all patients should be monitored with serial hCG determinations to diagnose and treat malignant sequelae promptly. Oral contraceptives have been demonstrated to be safe and effective during post-treatment monitoring based on randomized controlled trials. False-positive test results should be suspected if hCG values plateau at relatively low levels and do not respond to therapeutic maneuvers, such as methotrexate give for a presumed persistent mole or ectopic pregnancy. In compliant patients, the low morbidity and mortality achieved by monitoring patients with serial hCG determinations and instituting chemotherapy only in patients with post-molar gestational trophoblastic disease outweighs the potential risk and small benefit of routine prophylactic chemotherapy after evacuation of a molar pregnancy. Women with non-metastatic gestational trophoblastic disease should be treated with single-agent chemotherapy. For women with non-metastatic gestational trophoblastic disease, weekly dose of 30-50 mg/m2 of intramuscular methotrexate have been found to be the most cost-effective treatment when taking efficacy, toxicity, and cost into consideration. Women with high-risk metastatic gestational trophoblastic disease should be treated with multi-agent chemotherapy. This includes triple therapy with methotrexate, dactinomycin, and either chlorambucil or cyclophosphamide. More recent regimens further incorporate etoposide with or without cisplatin into combination chemotherapy. References: - Soper JT, Lewis JL Jr, Hammond CB. Gestational trophoblastic disease. In: Hoskins WJ, Perez CA, Young RC, editors. Principals and practice of gynecologic oncology. 2nd ed. Philadelphia (PA): Lippincott-Raven; 1997. p. 1039-77. (Level III)
- Curry SL, Schlaerth JB et al. Hormonal contraception and trophoblastic sequelae after hydatidiform mole. A Gynecologic Oncology Group study. Am J Obstet Gynecol 1989;160:805-9; discussion 809-11. (Level I)
- Kohorn EI. The new FIGO 2000 staging and risk factor scoring system for gestational trophoblastic disease: description and clinical assessment. Int J Gynecol Cancer 2001;11:73-7. (Level III)
- Kim DS, Moon H et al. Effects of prophylactic chemotherapy for persistent trophoblastic disease in patients with complete hydatidiform mole. Obstet Gynecol 1986;67:690-4. (Level I)
- Cole LA. Phantom hCG and phantom choriocarcinomas. Gynecol Oncol 1998;71:325-9.
- Felmate CM, Genest DR, Wise L, et al. Placental site trophoblastic tumor: a 17-year experience at the New England Trophoblastic Disease Center. Gynecol Oncol 2001;82:415-9 (Level II-2)
- ACOB Practice Bulletin No. 53. Diagnosis and treatment of Gestational Trophoblastic Disease. Vol. 103, No. 6, June 2004
- Homesley HD, Blessing JA et al. Weekly intramuscular methotrexate for non-metastatic gestational trophoblastic disease. A Gynecologic Oncology Group Study. Gynecol Oncol 1990;39:305-8 (Level II-2)
- Rustin GJ, Newlands ES et al. Weekly alternating etoposide, methotrexate, and actinomycin/vincristine and cyclophosphamide chemotherapy for the treatment of CNS metastases of choriocarcinomas. J Clin Oncol 1989; 7:900-3 (Level III)
- Soper JT. Gestational Trophoblastic Disease. Obstet Gynecol. 2006;108:176-187.
©
Women's Health and Education Center (WHEC)
|








