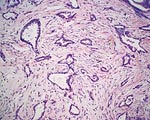
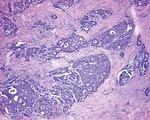
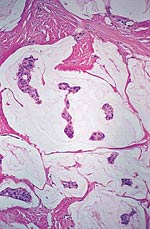
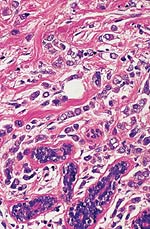
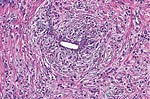
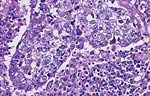
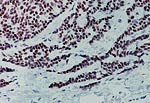
Pathology Of Breast CancerDr. Bruce R. Dziura Understanding the histopathologic features of breast cancer has been recognized as a necessary element for appropriate management of breast carcinoma. There have been two general approaches to prognostication via histopathologic analysis. The first categorizes carcinomas based on specific features, recognizing the so-called special-type carcinomas. The second approach evaluates individual characteristics of the carcinoma, such as nuclear pleomorphism or gland formation (grading). Recognizing the special-type carcinomas makes it possible to identify a group of patients with an extremely good prognosis, often approaching or equaling that of the general population. In contrast, a subset of patients who have a very poor prognosis can be identified (representing about 25% of invasive breast carcinomas) with careful histologic grading. The purpose of this document is to review the histopathology of invasive breast carcinoma, emphasizing the proven and potential settings in which it provides prognostic information. In-situ carcinomas of the breast were first recognized in the early 20th century and were identified morphologically as cells cytologically similar to those of invasive carcinomas but confined to duct structures. Short- and long-term risks associated with specific histologic variants or types of in-situ carcinomas are also discussed in this chapter. The terms ductal carcinoma in-situ (DCIS) and lobular carcinoma in-situ (LCIS) were once meant to signify separate anatomic origins -- one in ducts and the other in lobules. This concept is now accepted as an anachronism. Currently, the term ductal refers to patterns of abnormal epithelial cell proliferation associated with a prominent involvement of true ducts in the carcinoma in-situ (CIS) category and a high risk of local recurrence without adequate local treatment. The terminal ductal-lobular units (TDLUs) are a common anatomic site for the development of hyperplastic changes of both the ductal and the lobular type as well as corresponding neoplastic lesions (1). DCIS comprises of a heterogeneous group of non-invasive neoplastic proliferations with diverse morphologies and risks of subsequent recurrence and invasive transformation. The average age of DCIS is late 50s and 70% occurrence in postmenopausal women. Clinical signs are breast mass, Paget's disease and/ or nipple discharge. Mammographic signs -- microcalcification and risk of subsequent carcinoma is about 30%-50% at 10-18 years. Site of subsequent invasive carcinoma in the same breast is 99% and 1% in the other breast. Summary of DCIS Classification -- although nuclear grade and necrosis would appear to define most of the risk associated with DCIS, certain architectural patterns appear to carry significance independent of nuclear grade. High grade DCIS has extensive necrosis and high nuclear grade; Intermediate -- focal or absent necrosis with intermediate nuclear grade; Low grade -- absent necrosis with low nuclear grade. The literature on the multicentricity and multifocality of DCIS remains confusing because of the different definitions, methods of tissue processing and sampling techniques used as well as differences in the perspective of the investigators. High-grade DCIS lesions are at high risk for evolution to invasion and metastatic capacity and it is clear that extensive high-grade lesions are not easily cured and that recurrences are common even after radiation therapy. Careful pathologic assessment of DCIS lesions that includes histologic pattern, grade, size and margin status is essential for optimal clinical management and should be considered essential components of any breast biopsy report for DCIS. In 1941 Foote and Stewart introduced term LCIS and epithelial proliferative lesions (non-invasive or in-situ) of the human breast were termed lobular. Critical to definition and concept was the distinctive picture of lobular units produced by the diagnostic clustering of three major criteria: distension, distortion and filling by a population of characteristic cells. Also important to the definition in all probability, was that more than 60% of invasive cancer presenting with single filing of cancer cells with similar cytology have such in-situ lesions present in the same breast. It occurs in late 40s and 70% in premenopausal patients. Clinical signs may be none and mammographic signs are none specific. Risk of subsequent carcinoma is about 23%-30% at 15-20 years. Site of subsequent invasive carcinoma in the same breast is 50%-60% and in other breast 40%-50%. Thus lobular carcinoma in-situ (LCIS), lobular hyperplasia (LH) and atypical lobular hyperplasia (ALH) continue to signify an increased risk of later cancer development in either breast and other useful interactive associations remain to be evaluated fully (2). A current concern is the importance of finding ALH and/ or LCIS in core needle biopsies. There is full range of opinions with regard to the possible need for further excision with little apparent agreement. It is likely that extent of disease is important here and that several lobular units showing only standard ALH should not necessitate formal excision without other indications. There have been two general approaches to prognostication via histopathologic analysis. The first categorizes carcinomas based on specific features, recognizing the so-called special-type carcinomas. The second approach evaluates individual characteristics of the carcinoma, such as nuclear pleomorphism or gland formation (grading). Recognizing the special-type carcinomas makes it possible to identify a group of patients with an extremely good prognosis, often approaching or equaling that of the general population. In contrast, a subset of patients who have a very poor prognosis can be identified (representing about 25% of invasive breast carcinomas) with careful histologic grading. We support the histologic classification that recognizes special types of mammary carcinomas defined in terms of specific histologic criteria: lobular; tubular; medullary; and mucinous. In general, these special types are associated with less malignant potential than ordinary carcinomas that lack these special features. Tubular carcinoma of breast. The angulated shape of the glands and the cellular stroma are characteristic of this lesion. Probably the most important special type is tubular carcinoma because distant metastatic potential is highly unlikely when this tumor is present in pure form. The diagnosis is made when characteristic angulated tubules, composed of cells with low-grade nuclei, comprise at least 90% of the carcinoma. These neoplastic tubules are haphazardly arranged and are often found infiltrating between existing benign structures. Low-grade ductal carcinoma in-situ and atypical ductal hyperplasia are common findings. Tubular carcinoma has also been termed well-differentiated carcinoma, but this designation lacks precision, because carcinomas of no special type can also be well differentiated. Mammographic features include a speculated mass with or without associated microcalcifications or less commonly asymmetric density and architectural distortion with associated calcifications. Diagnostic features of tubular carcinoma seen in fine-needle aspiration specimens have been described (3). Closely related histologically and biologically to tubular carcinoma is invasive cribriform carcinoma (ICC). These carcinomas infiltrate the stroma as islands of cells that have the same appearance as cribriform-type ductal carcinoma in-situ. Differentiating cribriform in-situ from ICC may be difficult because of distortion and scarring. Irregular clustering of cellular islands signifies an invasive process. Another helpful feature is that the invasive islands in ICC are usually evenly spaced and often of uniform size. In studies of pure tubular carcinoma and ICC the presence of one or two positive low axillary lymph nodes did not adversely affect survival. The importance of pattern purity is emphasized because the presence of carcinoma that does not conform to special-type criteria increases the likelihood not only of nodal involvement but also of shorter survival. Mucinous carcinoma of the breast. Clusters of well-differentiated tumor cells are seen floating in a sea of mucin. Mucinous (colloid) carcinoma when present in its pure form is also associated with an excellent prognosis. Its defining histologic characteristic is extracellular pools of mucin in which low-grade tumor aggregates appear to be suspended. As with tubular carcinoma, the importance of pure patterns is essential to ensure an excellent prognosis (90% 10 year survival) in the absence of adjuvant chemotherapy (4). Pure and mixed mucinous carcinomas also have different mammographic appearances. Whereas pure mucinous carcinoma has a circumscribed, lobular contour on mammograms; mixed carcinomas have an ill-defined, irregular mammographic contour. This lack of circumscription corresponds histologically to the interface between invasive carcinoma and the often-fibrotic stroma. The two defining features of infiltrating lobular carcinoma (ILC) are cytology and pattern of infiltration. The classic ILC is composed of small cells with cytologic features identical to those of lobular carcinoma in-situ: regular, round, bland nuclei and cytoplasm with occasional intracytoplasmic lumina. Morphometric analysis of the nuclei in classic ILC shows small nuclear volume, adding a quantitative measure to the cytologic criteria. These cells infiltrate in single-file, frequently encircling existing structures (so-called targetoid pattern). When these two features are found in combination, the classic (or pure) pattern of ILC is diagnosed. It is this form of ILC that is most often associated with lobular carcinoma in-situ. Variant patterns have been described in which either the cytologic features or infiltrative pattern is present. These include the solid, alveolar, mixed and pleomorphic variants of ILC. They are often multifocal and bilateral, especially the pleomorphic variant. These features have little bearing on outcome either on overall survival or disease-free survival after conservative therapy. ILC are notorious for presenting diagnostic difficulties, clinically and radiographically. Although ILC is often associated with a discrete mass, a large proportion of these lesions are difficult to detect because of their insidious growth pattern. When compared with cancers of no special type but of similar size, patients of ILC have a better survival. Medullary carcinoma. The large tumor cells grow in a "syncytial" fashion and are sharply separated from the surrounding stroma, which is heavily infiltrated by lymphocytes and plasma cells. It has characteristic mammographic, clinical and pathologic correlates. Medullary carcinoma is a common phenotype of hereditary breast cancer and is found in women who are at risk for cancer because of mutations in the tumor-suppressor gene BRCA1. This genetic characteristic is in large part attributable to the young age of the patients (5). The distinctive smooth, pushing border of medullary carcinoma is reflected mammographically as a sharply circumscribed mass. Grossly, medullary carcinoma has a uniform, soft consistency. The essential histologic features include islands of tumor cells have irregular borders, without sharp edges, that are often connected (syncytial growth pattern). These islands do not invade the adjacent breast tissue, instead they appear to push against it resulting in a smooth interface with the adjacent normal breast tissue. In medullary carcinomas, the nuclei are large and pleomorphic with clumped chromatin, frequent nucleoli and readily identifiable mitotic figures. The other required histologic feature is a prominent infiltrate of lymphocytes and plasma cells in the loose connective tissue between the cellular islands. Unlike medullary carcinoma in other organs, medullary carcinoma of the breast is rarely associated with microsatellite instability. Node-negative medullary carcinoma predicts a good prognosis; otherwise the predictive utility is not clear. A better prognosis for the so-called medullary variant, or atypical medullary carcinoma, is not predicted beyond what would be expected for an ordinary intermediate-grade carcinoma of no special type. Approximately three fourths of invasive breast carcinomas do not have histopathologic features that would allow their inclusion into the aforementioned categories. These have been referred to as ductal because they do not have the lobular pattern described previously. Regardless of this common usage, these carcinomas have not been proved to arise in ducts, and in practice they are diagnosed through exclusion because they do not fit into any of the special-type categories. Designation -- no special type (NST) is also used. These NST carcinomas are characterized by a variety of patterns, from solid to small tightly cohesive nests to single-cell infiltrative patterns. Gland formation may be present, and often a mixture of these patterns is found in an individual carcinoma. These infiltrating NST carcinomas have the worst prognosis. They represent in total less than 2% of all breast carcinomas. Despite their rarity, specific diagnostic terms should be used in pathology reporting because of inherent clinical correlates. Secretory carcinoma: is usually small and well circumscribed. The characteristic histologic feature is the presence of abundant intracellular and extracellular clear areas that contain secretions. The secretory material stains with periodic acid-Schiff stain and other muco-substance stains. It is usually seen in younger patients, but can also affect older women. Features that ensure an excellent prognosis include young age, tumor diameter less than 2 cm, and no stromal invasion at the periphery of the lesion (6). Squamous cell carcinoma: it may be a feature of NST carcinomas; pure pattern of squamous cell carcinoma is distinctly unusual. Often cystic, squamous cell carcinoma of the breast may also assume a solid pattern with keratinization. The importance in its recognition lies with better understanding in the event of later metastases. Prognosis of squamous cell carcinoma of the breast is probably the same as that for ordinary intermediate to high-grade carcinoma NST. Rarely, breast carcinoma displays the same pattern as well-accepted salivary gland tumors. The most important salivary gland type of tumor in the breast is adenoid cystic carcinoma because of potential confusion in prognosis. Mucoepidermoid carcinoma of the breast has the same histopathologic features as the salivary gland counter-part. The salient features -- mucin production and squamous differentiation can however, be a non-specific feature of mammary carcinoma NST. The term metaplastic carcinoma encompasses a group of tumors that show both epithelial and mesenchymal features. The mesenchymal component may show squamous, spindled, cartilaginous, or osseous differentiation. Immunocytochemical stain for estrogen receptors in invasive breast carcinoma. The strong nuclear positivity in tumor cells is shown against a negative cytoplasmic and stromal background. The immuno-histochemical determination of estrogen receptor (ER) in paraffin-embedded tissue yields results that closely parallel biochemical determinations. These tissue section analyses are advantageous for the following reasons: (i) the same tissue that is used for making the diagnosis is used for the ER analysis; thus tissue is conserved. (ii) The presence of ER is detected in the context of histopathology. Thus the physician is ensured that the positive signal detected is emanating from carcinoma instead of benign epithelium. The biochemical assay is based on a tissue homogenate, so it is impossible to know which cells are responsible for positive signal. This is also an issue if carcinoma in-situ is assayed biochemically, rather than the invasive component. (iii) Greater sensitivity is achievable with the immuno-histochemical assay. If a carcinoma is not very cellular or if there are many contaminating stromal or inflammatory cells, a resulting dilutional effect may be encountered in the biochemical assay. In contrast, positive cells even if very rare are easily recognized immuno-histochemically. Although the clinical predictive power of the biochemical method has been well established, the immuno-histochemical method for determining ER status is superior in predicting response to adjuvant endocrine therapy (7). In general, lymphatics are the vessels most often involved in breast carcinoma, although blood vessels sometimes contain carcinoma. Misinterpreting carcinoma in soft tissue spaces or Intraductal carcinoma as tumor in lymphatic spaces is often responsible for the overdiagnosis of lymphatic invasion. These mistakes may be avoided if the physician requires the presence of tumor cell emboli in a space lined by endothelial cells to make the histologic determination of vascular invasion. Carcinoma involving the lymphatic spaces of the dermis often results in the distinctive clinical entity inflammatory breast carcinoma. This diagnosis is made when a breast containing carcinoma is red, edematous, and warm. It is rare for patients with inflammatory carcinoma to survive 5 years, although a multimodality approach -- multiagent chemotherapy, surgery and radiation may improve survival (8). In summary, prognostication based on tumor stage, histologic recognition of special types of carcinomas and careful histologic grading are proven predictive factors. Surgical pathology reporting is incomplete without them. Studies of new prognostic factors should include these proven factors in the final analysis of independent significance. Open biopsies from breast lesions are usually of excisional type when the tumor measures 2.5 cm or less It should be added here that intraoperative cytologic examination can be very useful and that this procedure is used routinely by some authors in conjunction with (or instead of) the frozen section procedure. When interpreted by experienced individuals, the smears are as accurate as the frozen sections. Finally, frozen sections have been used effectively in evaluating re-excision lumpectomy margins. With the development of the Cell Search System, it has become possible to measure circulating tumor cell (CTC) levels with high reproducibility, and the CTC test is currently being used clinically for patients with metastatic breast cancer in the United States. Circulating tumor cells (CTCs) can be detected in the peripheral blood of around 50% of patients with metastatic breast cancer. Their numbers are an independent predictor of the patient's Understanding DCIS of the breast has been a consistent challenge because of changing presentations of DCIS and changing concepts and therapeutic options. It is impossible to understand DCIS without a historical background. The terms and concepts developed over the last 10 years seem to be serving us nicely for most presentations of DCIC in high- and low-risk categories with precise categorizations with the intermediate, and special-type categories to await further study. Multicentricity and bilaterality have sometimes been considered related in some aspects. Difficulties in defining the relationships of multiple tumors should not obscure the recognition that it is important only as it had predictive value for treatment success or failure. True multicentricity is defined as independent index sources of tumor. It is difficult to estimate and its occurrence is much less often than has been published. |