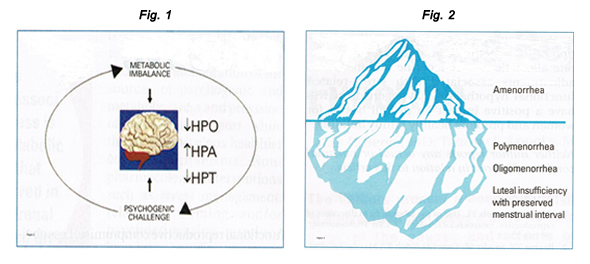
وسائل منع الحمل والصحة النفسية[وهك] ممارسة نشرة وسريرية إدارة لمقدمي الرعاية الصحية.قدمت منحة للتربية وصحة المرأة وتربية مركز ([وهك]). By the year 2020, the World Health Organization (WHO) estimates that depression will rank second only to cardiovascular illness in terms of disease burden and as a worldwide cause of disability. Among the most prevalent and disabling chronic diseases affecting reproductive-aged women worldwide, depression and anxiety can contribute to adverse reproductive health outcomes, including an increased risk of unintended pregnancy and its health and social consequences. Women bear an unequal burden of mental illness. The existence of comorbid mental and medical illnesses influences management strategies whether women require prenatal care or treatment for postpartum depression, therapy for low sexual desire, relief from menopausal symptoms, or treatment for the menstrual disorders. Sharing knowledge and best practices across specialties is one way to improve mental illness. Hormonal fluctuations occurring at key points in the female life cycle - during puberty, pregnancy and post-pregnancy, perimenopause, early postmenopause and old age - put some groups of women at greatly increased risk of major depression is linked to a large increase in mortality (about 15%) and health problems, especially metabolic disorders. Low libido and other problems in sexual functioning are unfortunate side effects of anti-depression and anti-anxiety medications. Primary care healthcare providers and obstetricians and gynecologists who recognize potential mental health issues and are familiar with the mental health issues are well suited to provide effective and efficient patient care. The purpose of this document is to discuss and manage women with the common mental health conditions who want to avoid an unplanned pregnancy. Effective contraception can be an important strategy to maintain and even improve mental health and well-being. Reproductive health clinicians play a critical role in providing and managing contraception to help women with mental health considerations achieve their desired fertility. This discussion reviews the literature on relationships between mental health and contraception and describes considerations for the clinical management of contraception among women with depression and anxiety. The issues related to contraceptive method effectiveness, adherence concerns, and mental health - specific contraceptive method safety and drug interaction considerations, clinical counseling and management strategies are also discussed. Given important gaps in current scientific knowledge of mental health and contraception, the Women's Health and Education Center (WHEC) highlights areas for future research. Ultimately, mental health promotion may reduce adverse pregnancy-related outcomes, improve family-planning experiences, and help achieve reproductive goals for women, their families, and society. BackgroundDepressive and anxiety disorders are among the leading causes of disability in the United States and worldwide (1). Compared with men, women in United States are 70% more likely than men to experience a depressive disorder, and 60% more likely to experience an anxiety disorder (1). Approximately 12% of women will experience major depression in their lifetime; 8.4% will experience a depressive disorder each year (1). Women experience anxiety disorders at even higher rates, and anxiety disorders are highly comorbid with depression. And although common, depression and anxiety disorders often go undetected and untreated among reproductive-aged women. In recent years, less than half of US women aged 15-44 years with a major depressive episode received a mental health diagnosis and less than half received treatment (2). Moreover, poor, unemployed and less educated women experience higher rates of mental health disorders and lower rates of mental health detection and treatment than their socially advantaged counterparts; racial/ethnic minority women receive mental health care at even lower rates. Mental Health, Unintended Pregnancy and Reproductive OutcomesWomen with depression and anxiety experience and elevated risk of unintended pregnancy, and those pregnancies may be more likely to end in induced abortion, compared with women without depression and anxiety (3). Depression and anxiety are precursors to a host of negative perinatal and postpartum outcomes, including maternal and infant morbidity, obstetrical complications, preterm labor, stillbirth, low birth-weight, and antepartum and postpartum depression, especially when pregnancies are unintended (4). Poor, underinsured, undereducated, and minority women disproportionately suffer mental health morbidity, low rates of detection and treatment, and adverse reproductive outcomes, including unintended pregnancy. Stress and Reproductive FunctionStress is the most common and under-appreciated cause of reproductive compromise, including menstrual cycle disturbances and amenorrhea, infertility, and preterm labor. Acute stress elicits transient neuro-endocrine, metabolic, and behavioral responses to facilitate homeostatic survival adaptations. Chronic stress provokes more sustained or "allostatic" adjustments in these same physiologic systems. Although allostatic adaptations likely promote survival as well in the short term, they also engender detrimental consequences in the long term. Physical sequelae of long-term stress include osteoporosis, depression, hypertension, and cardiovascular disease, as well as accelerated aging and dementia. Gonadal function depends directly on hypothalamic gonadotropin-releasing hormone (GnRH) drive. Decline in endogenous pulsatile GnRH secretion reduces both luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels. Reduced GnRH drive compromises folliculogenesis, and may result in luteal insufficiency or anovulation. Decreased GnRH pulsatility has been shown to cause anovulation and amenorrhea (5). Decrements in central GnRH-LH drive comprise a continuum, varying from day to day to cause a spectrum of ovarian disorders. Figure 1: Psychogenic challenge and metabolic imbalance interact synergistically to suppress ovarian function, activate adrenal secretion of cortisol, and suppress thyroidal secretion of thyroxine. Figure 2: Continuum of hypothalamic hypogonadism in women, with amenorrhea representing only the tip of the iceberg as related to the incidence of functional forms of hypothalamic hypogonadism. Amenorrhea, polymenorrhea, or oligomenorrhea are more obvious but represent only a small proportion of the spectrum of functional reproductive compromise. Less obvious forms and more common forms include luteal phase deficiency with preserved menstrual interval with normal luteal phase length but decreased overall progesterone secretion. The most common cause of reduced GnRH drive is functional; that is, it is not due to organic causes such as hypothalamic tumors or pituitary adenomas, but rather to functional hypothalamic hypogonadism. In this theoretically reversible form of gonadal compromise, psychophysiological and behavioral responses to life stressors activate central neuro-regulatory networks, evoking concomitant metabolic mobilization and reproductive suppression due to disruption of GnRH secretion (6). The variables that activate the adrenal axis, suppress the thyroidal axis, suppress the thyroidal axis, and halt ovulation are not always readily identifiable. Psycho-social stressors activate the central pathways of perception, whereas exercise and weight loss present metabolic challenges. However, there is no method for clearly differentiating psychogenic from metabolic stress. Psychogenic stress has a metabolic cost, and metabolic stressors (e.g. food restriction, excessive exercise) are often initiated to cope with psychogenic stress. The mainstays of therapy for women with stress-induced anovulation are oral contraceptives when fertility is not desired, and ovulation induction or assisted reproduction when it is. The rationale for these interventions is based on the view that stress-induced anovulation represents isolated compromise of reproductive manifestations need to be treated. Several lines of research invalidate this perspective, including the association of stress-induced anovulation with constellation of neuroendocrine aberrations. The more clinically evident the ovarian compromise, the greater the hypothalamic disruption, and the more profound the associated adrenal and thyroid derangements and sex steroid deprivation. For a woman with functional hypothalamic hypogonadism seeking conception, ovulation induction can be accomplished via exogenous pulsatile GnRH therapy or gonadotropins. Essentially, the stress process must be interrupted. The most common treatment for a woman with stress-induced anovulation not seeking immediate conception is hormonal and oral contraceptives are the first line of management. The optimal intervention is to reverse the stress process so that the hypothalamus recovers and gonadal function resumes. Ideally, treatment approaches that dampen the activation of stress pathways and reverse or ameliorate allostatic neuroendocrine and metabolic adjustments associated with stress-related functional hypothalamic hypogonadism will have a positive impact on overall health for women and promote healthy family building. Women with depression and anxiety experience an elevated risk of unintended pregnancy, those pregnancies may be more likely to end in induced abortion, compared with women without depression and anxiety (3). Depression and anxiety are precursors to a host of negative perinatal and postpartum outcomes, including maternal and infant morbidity, obstetrical complications, preterm labor, stillbirth, low birth-weight, and antepartum and postpartum depression, especially when pregnancies are unintended. Poor, underinsured, undereducated, and minority women disproportionately suffer mental health morbidity, low rates of detection and treatment, and adverse reproductive outcomes, including unintended pregnancy. Deficiencies in neurotransmitters that have an impact on mood (serotonin, norepinephrine, dopamine, γ-aminobutyric acid, and peptides) have been implicated in clinical studies of depression and anxiety, and genetic predisposition and psychosocial stressors appear to be important precursors to neurotransmitter deficiencies (1). Contraceptive researchers in the 1960s and 1970s hypothesized that large dosages of synthetic estrogens and progestins in combined oral contraceptive pills (e.g. 5 mg norethynodrel, 75 μg mestranol) could potentially interact with mood-related neurotransmitters and neurotransmitter metabolism (7). Although there have been no published clinical trials to date using hormonal bioassays or brain imaging to clarify these relationships, newer evidence suggests that the steroidal activity of lower-dosage modern contraceptives do not have a clinically relevant physiological impact on women's mood or mood-related neuroendocrine functioning. In a systematic review of studies examining combined oral contraceptive pharmacological properties and mood, there was no evidence for an association between the intrinsic biochemical mechanisms of combined oral contraceptives and mood side effects reported by the users (8). In the 2010 Medical Eligibility Criteria for Contraceptive Use report, the Centers for Disease Control and Prevention (CDC) concluded there are no contraindications to hormonal contraception for women with depression, citing a lack of evidence supporting a causal relationship. Prospective population-based cohort studies and clinical placebo-controlled trials have consistently reported similar or even lower rates of depression or mood symptoms in combined oral contraceptive users compared with non-users (7). More recent pharmacological research on fourth-generation drospirenone-containing combined oral contraceptives found improvements of premenstrual dysphoric disorder mood symptoms. Research on the depot medroxyprogesterone acetate (DMPA), transdermal patch, vaginal ring, sub-dermal implant, and levonorgestrel-releasing and copper-containing intrauterine devices (IUDs) has also found no evidence of negative mood effects with the use of these methods (9). Given that some recent studies have relied upon observational and cross-sectional designs and small sample sizes, additional research that uses rigorous prospective, longitudinal, and randomized controlled trial designs is needed to provide a more definitive comment on the null effects of contraception on women's mental health. A growing number of studies have documented higher rates of contraceptive non-use, misuse, and discontinuation among women with depressive, anxiety, and related stress and distress symptoms compared with women without symptoms. These findings, which have been consistent across studies, populations, and settings, have been most widely noted for combined oral contraceptives and condoms but have also been demonstrated for DMPA, IUDs and implants (9). The impact of mental health on contraceptive method selection is less clear. Some clinical and population-based studies of non-pregnant women have found less effective method use (i.e. condoms and withdrawal vs. combined oral contraceptions and long-acting reversible contraception) to be associated with higher depression and stress symptoms, where as a study of post-abortion patients found higher rates of IUD use was associated with greater mental distress symptoms (10). Reasons for these differences across contexts are not fully apparent and warrant further research. Little science exists to explain how or why mental health influences contraceptive behavior. Psychological research suggests that altered cognitive processes may contribute to heightened perception of physical symptoms among women with mental health conditions. Combined oral contraception discontinuation rates from perceived mood symptoms are not uncommon (range, 14% - 21% in some studies), despite the evidence refuting causal associations (11). Depressed or anxious women may also internalize negative or incorrect information about contraception and have exaggerated concerns about risks and side effects. Additionally, risk assessment, planning, social learning, decreased motivation, and desire for self-care, excessive worry, and diminished perceptions of susceptibility to pregnancy may have an impact on contraceptive decision-making processes and lead to sub-optimal contraceptive choices among women with depression and anxiety. Additional studies are needed to test these mechanistic theories. In busy clinical settings, standardized mental health screening is an efficient, effective, and feasible way to improve detection of depression and anxiety and thus should be used routinely and systematically, including with all well-woman examinations and new patients. The Prime-MD Patient Health Questionnaire (PHQ) is perhaps the most commonly used, preferred screening tool in current primary care and other non-psychiatric settings and can be seamlessly included in electronic medical record charting. Table below highlights commonly used, evidence-based screening instruments (12): Differential Diagnosis for new-onset mental health symptoms may include hypothyroidism, diabetes mellitus, anemia, cancer, multiple sclerosis, eating disorders, substance abuse disorders, acute stress or grief, or use of certain medications such as beta-blockers or calcium channel blockers, glucocorticoids, or gonadotropin-releasing hormone agonists. These conditions can cause symptoms that mimic depression or anxiety and should be ruled out (13). Provision of basic information on the prevalence, signs, symptoms, and treatment options for depression, anxiety, and related disorders may facilitate an open dialog between the patient and healthcare provider. Referral systems should be in place for women who require further evaluation and treatment by a mental health specialist. For the most part, women with depression and anxiety are eligible for the full range of contraceptive methods, and method selection should occur through shared decision making between the patient and provider, taking into account individual health circumstances, contraceptive preferences, and fertility goals. In the CDC Medical Eligibility Criteria for Contraceptive Use, depression is a category 1, indicating no restrictions for use of any form of hormonal contraception (14). Modern pharmacological antidepressant agents, including the most commonly used selective serotonin reuptake inhibitors (SSRIs; such as fluoxetine, citalopram, Escitalopram, and sertraline) and serotonin norepinephrine reuptake inhibitors (SNRIs; such as venlafaxine and duloxetine), do not appear to interact with hepatic metabolism of hormonal contraceptives, including combined oral contraceptives (15). In contrast, tricyclic antidepressants (TCAs; such as amitriptyline or nortriptyline) and monoamine oxidase inhibitors (MOIs; such as phenelzine and tranylcypromine), which are older-generation antidepressant agents used in treatment-refractive chronic depression, are highly interactive with foods and drugs and may interact with contraceptive steroid metabolism in the liver, potentially leading to decreased contraceptive efficacy or antidepressant side effects or toxicity (16). St. John's wort (hypericum perforatum), and over-the-counter antidepressant therapy, may also induce the cytochrome P450 system and subsequently reduce contraceptive steroid availability, although existing evidence is inconsistent (17). For women who require more intensive psychiatric treatment with TCAs, MAOIs, or those using St. John's wort, locally acting methods such as the levonorgestrel-releasing IUD or copper-containing IUD are safe alternatives. Non-pharmacological therapies, including cognitive behavioral therapy, interpersonal psychotherapy, and adjunct therapies like exercise, sleep, and healthy diet, should not interfere with contraception or preclude the use of any methods and may reinforce problem-solving and coping skills and self-care techniques that mutually benefit family-planning decision making and behaviors. Potential contraceptive adherence issues may be an important factor for appropriate method selection for women with depression and anxiety. The greatest body of evidence linking mental health and contraceptive side effects, misuse, and discontinuation has been documented for user-dependent methods, including condoms and combined oral contraceptives (15). Long-acting reversible contraception methods require little user burden, are cost effective, and offer the greatest contraceptive effectiveness, potentially making them ideal options for women with mental health conditions (18). For women who do not prefer long-acting methods, DMPA, the vaginal ring or transdermal patch are alternative options. For combined oral contraception selection and management, several strategies may be clinically useful. Research has suggested that women's experiences with perceived side effects, including mood, are similar across different types of combined oral contraceptives, and most women do well the any combined oral contraceptive (19). In early high-dose combined oral contraception formulations, a dose-response relationship with mood side effects was noted (18). So although newer research does not support such a relationship with modern doses, providers may consider formulations with lower estrogen dosages (i.e. 20 μg vs. >20 μg). This should be balanced with the likelihood of other side effects, including irregular bleeding as well as potentially reduced effectiveness with missed pills (20). Widely used levonorgestrel- and norgestrel-containing combined oral contraceptives are not known to contribute to mental health symptoms, and some research has shown improved mood symptoms among women taking second-generation combined oral contraceptives compared with women on placebo (20). Newer drospirenone-containing combined oral contraceptives are approved by the Food and Drug Administration (FDA) for the treatment of mood symptoms occurring specifically with premenstrual dysphoric disorder (PMDD) mood disorder and should be an option for women with depression and anxiety. Additionally, cyclic mood symptoms occurring during ovulation or menses may respond well to steady dose monophasic vs. multiphasic formulations (21). Estrogen withdrawal mood symptoms during combined oral contraceptive placebo weeks may respond to extended cycle regimens (e.g. 24/4, 84/7) or continuous dosing (i.e. skipping inactive pills). In specific cases, such as postpartum and perimenopausal depression, women may not be aware of their risk of unintended pregnancy and may also have unique contraceptive considerations. Estrogen-containing contraceptives are generally not recommended during the first postpartum month among breastfeeding women because of possible reduction in breast milk production (18); instead, the copper-containing IUD or progestin-only methods including the levonorgestrel-releasing IUD, subdermal implant, DMPA, or progestin-only pills can be used. For ovulating perimenopausal women with hormonal and mood fluctuations or menstrual and vasomotor symptoms, combined oral contraceptives, the patch, or the ring may also treat symptoms. Women's cardiovascular risks, tobacco use, and other contraindications to estrogen should be assessed. Women with depression and anxiety, as well as all women, may benefit from education and counseling strategies that deliver repeated and specific information on user-related method effectiveness rates and the relationship of contraceptive effectiveness to mental well-being (e.g. daily pills require diligence and motivation for correct use, with which depression or anxiety may interfere) (22). Healthcare providers should monitor for the counsel on adherence issues; reports of missing dosages or discontinuation of an antidepressant may alert the provider to contraceptive misuse or discontinuation. Evidence-based counseling techniques like motivational interviewing can be used to focus attention on specific problematic behaviors (e.g. frequently missed pills, circumstances around condom nonuse) to evoke motivation for change by increasing confidence, readiness, and planning to improve contraceptive behaviors (for instance, the use of cell phone reminders or a consistent daily pill-taking routine). Healthcare providers should also work to dispel myths and misperceptions of side effects and reinforce the benefits and positive non-contraceptive effects of modern methods. Finally, counseling should include information on dual-method use (i.e. condoms) and sexual risk behavior given documented higher rates of sexually transmitted infections among women with depression and anxiety (23). An assessment of other dimensions of women's lives that may have an impact on mental and reproductive health, including financial hardship, lack of social support, relationship violence, and other stressful life events may identify potential triggers of depression and anxiety, especially among socially disadvantaged groups of women who disproportionately experience these risk factors. Women in violent or controlling relationships may experience reproductive coercion, birth control sabotage, intentional exposure to sexually transmitted infections, unintended pregnancy, and lack of control over their pregnancy outcomes; they may also have limited access to health services and contraception (24). Routine screening for intimate partner violence can identify women who may have special contraceptive and mental health considerations. Inconspicuous methods that maximize contraceptive control and effectiveness and minimize the likelihood of exacerbating partner resistance or violence, such as DMPA, may be helpful. IUDs or the implant may offer other subtle highly effective options that require less frequent health service visits. Information on local resources for counseling or mental health services and insurance or medication assistance programs should be readily available in reproductive health clinical settings. Sign and symptoms of depression and anxiety can have an impact on patient-provider communication and interaction in important ways but may not be obvious to the provider or patient. Lack of awareness and insight into mental health issues and perceived stigma are barriers to disclosure of mental health symptoms. Assessment of psychological well-being and its impact on sexual and reproductive health functioning should be a routine component of the patient interview. Patience, empathy, and use of reflective listening, a non-judgmental tone, and open-ended questions may facilitate women's comfort in disclosing mental health issues. Discussions can be initiated with an educational statement such as, "Did you know that more than one fifth of women will have symptoms that meet criteria for depression in their life time? Because depression is so common, I like to check in with all my patients about their own mental health". Observing for a sad voice, anxious expressions, lethargic posture, or a clinical presentation of multiple, vague complaints, non-specific symptoms, or pain-related syndromes (e.g. non-specific vulva, pelvic, vaginal, coital, or menstrual-related pain, headaches, or gastrointestinal disturbances) may alert providers to an underlying depressive or anxiety disorder. Healthcare providers should also monitor for transference: feeling down, sad, and upset after seeing a distressed patient. Depression and anxiety are common among reproductive-age women and have significant implications for reproductive health, including the risk of unintended pregnancy and adverse perinatal and postpartum outcomes. There has been a lack of attention to mental health in family planning contexts, and significant research gaps exist and preclude an in-depth understanding of the most effective approaches for contraceptive management among women with depression and anxiety. Although additional research can more comprehensively identify the role of mental health in contraceptive management (and vice versa), reproductive health providers should prioritize contraceptive counseling and management for women with depression and anxiety who want to avoid unintended pregnancy. Ultimately, mental health promotion may reduce adverse pregnancy-related outcomes, improve family-planning experiences, and help achieve reproductive goals for women, their families and society. |