Síndrome de muerte súbita infantilWHEC Boletín Práctica Clínica y Gestión de los proveedores de salud. Educación subvención prevista de Salud de la Mujer y el Centro de Educación (WHEC). Significant new information has been forthcoming in recent decades on sudden infant death syndrome and apnea during early infancy. Sudden Infant Death Syndrome (SIDS) also known as Sudden Unexpected Infant Death (SUID) and Sudden Unexpected Death in Infancy (SUDI), are the terms used to describe unexpected death of an infant less than 12 months of age. The cause of death that cannot be explained after thorough investigation, death scene examination, and review of clinical history. These deaths often happen during sleep or in the baby's sleep area. It is well established that environmental and biological risk factors contribute to SIDS. There is also growing consensus that SIDS requires the intersection of multiple risk factors that result in the failure of an infant to overcome cardio-respiratory challenges. Thus, the critical next steps in understanding SIDS are to unravel the physiological determinants that actually cause the sudden death, to synthesize how these determinants are affected by the known risk factors, and to develop novel ideas for SIDS prevention. More than 25 years have elapsed since continuous cardio-respiratory monitoring at home was suggested to decrease the risk of SIDS. In the ensuring interval, multiple studies have been unable to establish the alleged efficacy of its use. In this statement, the most recent research information concerning extreme limits for a prolonged course of apnea of prematurity is reviewed. Recommendations regarding the appropriate use of home cardio-respiratory monitoring after hospital discharge emphasize limiting use to specific clinical indications for a predetermined period, using only monitors equipped with an event recorder, and counseling parents that monitor use does not prevent sudden, unexpected death in all circumstances. The continued measures are encouraged. The purpose of this review is to explore current and emerging perspectives related to cardio-respiratory dysfunctions in SIDS. Specifically, the potential relationship between SIDS, auditory function, and central chemosensitivity are explored. It underscores the importance of combining the epidemiological and pathological determinants of this syndrome. The efforts of the Women's Health and Education Center (WHEC) is to improve better understanding of this syndrome which may lead to novel ways to reduce the risk to succumb to SIDS. Introduction and DefinitionsApnea of infancy is defined as "an unexplained episode of cessation of breathing for 20 seconds or longer, or a shorter respiratory pause associated with bradycardia, cyanosis, pallor, and/or marked hypotonia." The term "apnea of infancy" generally refers to infants with gestational age of 37 weeks or more at the onset of apnea (1). Apnea of prematurity is defined as sudden cessation of breathing that lasts for at least 20 seconds or is accompanied by bradycardia or oxygen desaturation (cyanosis) in an infant younger than 37 weeks' gestational age. It usually ceases by 37 weeks' postmenstrual age but may persist for several weeks beyond term, especially in infants born before 28 weeks' gestation (1). The most recent data indicate that extreme episodes usually cease at approximately 43 weeks' postconceptional age. SIDS is not the cause of every sudden infant death. Each year in the United States, thousands of infants die suddenly of no immediately obvious cause. These deaths are classified as SUID. SUID is the death of an infant younger than 1 year of age that occurs suddenly and unexpectedly. SUID includes all expected deaths: those without a clear cause, such as SIDS, and those from a known cause, such as accidental suffocation. Many unexpected infant deaths are accidents, but a disease or another external factor, such as poisoning or neglect, can also cause an infant to die unexpectedly. One-half of SUID cases are SIDS (1). Sleep-related causes of infant death are those linked to how or where a baby sleeps or slept. They are due to accidental causes, such as: suffocation; entrapment, when baby gets trapped between two objects, such as a mattress and wall, and can't breathe; or strangulation, when something presses on or wraps around baby's neck, blocking baby's airway. These deaths are not SIDS. While the precise cause of death is unknown, it is well-established that SIDS correlates with infant sleep position and deficiencies in cardio-respiratory function (2). The intense efforts to better understand the etiology of SIDS has led to the development of a triple risk model involving:
According to this generalized model, the intersection of these risk factors leads to a significant increase in the chance of SIDS. While two of the three risk factors are intimately related to the biology of the infant, the third factor, an exogenous stressor, recognizes the important role environment plays in the pathogenesis of the syndrome. Perhaps the most recognized exogenous stressor correlating to SIDS is an infant sleeping in the prone position. In 1996, the United States launched the Back-to-Sleep campaign to better educate the public about the potential danger of infants sleeping in the prone position. This campaign dramatically reduced the occurrence of SIDS (4). However, despite the success of this campaign, SIDS remains a leading cause of infant death. Furthermore, a recent report finds that although the relative contributions of risk factors for SIDS have changed, the majority of SIDS cases still involve both, intrinsic and extrinsic risk factors (5). An apparent life-threatening event (ALTE) is defined as "an episode that is frightening to the observer and is characterized by some combination of apnea (central or occasionally obstructive), color change (usually cyanotic or pallid but occasionally erythematous or plethoric), marked change in muscle tone (usually marked limpness), choking, or gaging". (6). Prevalence of SIDS and SUIDSUID Registry started in 2009 and SUID data is not linked to DNA sample. In 2017, in the United States, there were 3,600 SUID. These deaths occur among infants less than 1 year old and have no immediately obvious cause (7). The three commonly reported types of SUID include the following:
In 2017, there were about 1,400 death (38%) due to SIDS, about 1,300 deaths (36%) due to unknown causes and about 900 deaths (26%) due to accidental suffocation in bed. The SUID rate, which includes SIDS, unknown causes, and accidental suffocation and strangulation in bed (ASSB), declined in USA considerably beginning in 1990 (see figure 1 below). This decline followed the release of various recommendations by The American Academy of Pediatrics (AAP) and initiation of various Safe to Sleep programs. Trends in SUID by cause, 1990 – 2017 in the United States 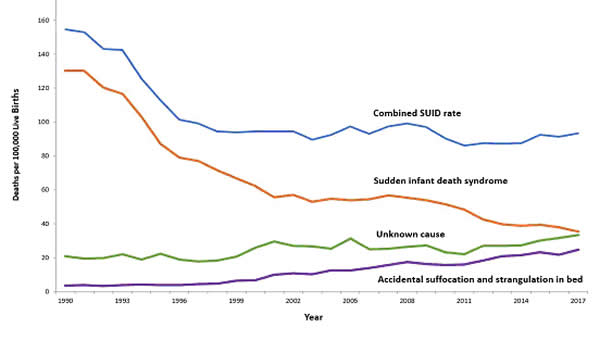 Since 1999, declines have slowed. In 2017, the SUID rate was 93.4 deaths per 100,000 live births. In recent years, SUID are being classified less often as SIDS, and more often as accidental suffocation or strangulation in bed or unknown cause. SIDS rates declined considerably from 130.3 deaths per 100,000 live births in 1990 to 35.4 deaths per 100,000 live births in 2017. Unknown cause infant mortality rates remained unchanged from 1990 until 1998, when rates began to increase. In 2017, the unknown cause mortality rate in infants was 33.4 deaths per 100,000 live births. Accidental suffocation and strangulation in bed mortality rates remained unchanged until the late 1990s. Rates started to increase beginning in 1997. In 2017, the rate was 24.6 deaths per 100,000 live births. SUID deaths by race/ethnicity: SUID rates per 100,000 live births for American Indian / Alaska Native (205.8) and non-Hispanic black infants (181.0) were more than twice those of non-Hispanic white infants (85.0). SUID rates per 100,000 live births were lowest among Hispanic (52.2) and Asian/Pacific Islander infants (33.5). Deaths due to SIDS accounted for the largest proportion of SUIDs for most racial / ethnic groups, ranging from 40% of SUID among Hispanic infants to 47% of SUID among American Indian / Alaska Native infants. Accidental suffocation and strangulation in bed accounted for the smallest proportion of SUID for all racial groups, ranging from 20% of SUID among Hispanic and Asian / Pacific Islander infants to 27% of SUID among non-Hispanic black infants (7). Patho-Physiology of SIDSOne of the biggest puzzles in understanding SIDS is why children that succumb to SIDS appear to breath normally under control conditions, yet fail to adequately sigh, gasp, autoresuscitate, and arouse during an environmental challenge. What we need to understand are the mechanisms that differentiate gasping and sighing from those of normal breathing. Without understanding these differences, we will be unable to identify vulnerable infants before it is too late. In the brainstem, central neural networks integrate sensory inputs, generate respiratory rhythms, and produce coordinated motor output in order to control the timing, amplitude, and duration of each breath. This is true for eupnea, sigh, and gasping behaviors although initially sighs and gasps were thought to be simple reflexes (8). Breathing behavior emerges through the integrations of several neural populations within the ventral respiratory column including the preBÖtzinger complex (preBÖtC), BÖtzinger complex, Retrotrapezoid Nucleus / Parafacial respiratory group and also the KŐlliker-Fuse as well as some cortical and cerebellar networks (9). A popular view was that different types of breathing activities were produced by separate and distinct centers. Many believed that so-called pneumotaxic center exists in the pons while a gasping center was thought to be located in the ventrolateral medulla (10). This view was based on simple lesion experiments. This view has been challenged by future authors. Moreover, the concept that separate centers generate different forms of breathing was changed by a much more plastic concept, i.e., that different forms of breathing are generated by network reconfiguration. Specifically, this concept proposes that the same network can generate more than one breathing pattern by undergoing a network reconfiguration. This study demonstrated that the preBÖtC, even when isolated in a brainstem slice, is capable of producing neural rhythms fundamental to the generation of three significantly different respiratory activities: eupnea, sighs, and gasps (11). The concept of network reconfiguration is a significant conceptual departure from the contemporary understanding of its time. The implications of this conceptual change are important to understanding the physiological determinants of SIDS. The preBÖtC is a multi-functional network critical for generating the respiratory network to hypoxia (12). Even in the reduced state of an isolated brainstem slice, the preBÖtC is dynamically responsive to hypoxia and reoxygenation. The preBÖtC is comprised of a heterogenous population of neurons that can be readily identified in synaptic isolation: non-pacemaker neurons, such as autonomous spiking neurons. Sighs, gasps and the arousal response Recognition that the prone position is a major SIDS risk factor has not only saved lives through the Back-to-Sleep campaign but also provides critical mechanistic insights into physiological determinants of SIDS. The insights gained are consistent with a final common pathway of cardio-respiratory distress that SIDS victims experience involving arousal and/or auto-resuscitation deficiencies. Specifically, an infant sleeping in the face-down position re-breaths exhaled gases which leads to a build-up of inspired CO2 with a concomitant decrease in inspired O2 supply of the infant (13). Under normal conditions increased CO2 (i.e. hypercapnia) is a physiological stimulus that leads to a stereotypical arousal response that relieves the buildup of end-tidal CO2 and refreshes the inspired O2 supply of infant. This arousal response constitutes a powerful mechanism that normally protects an infant from respiratory distress associated with the prone position. 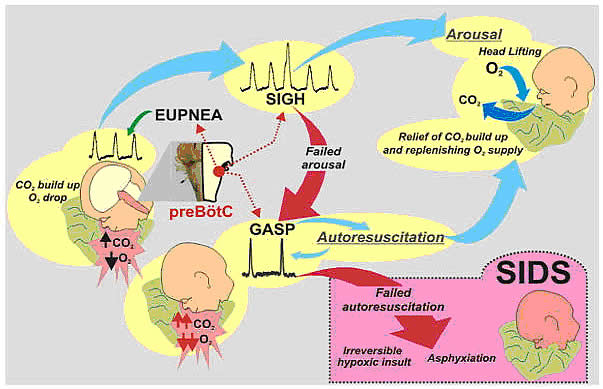 However, prospective studies have shown that spontaneous and induced arousals during sleep are decreased in SIDS victims indicating an innate susceptibility was present before the final event (14). Thus, any factor that blunts the initiation or effectiveness of the arousal response may increase the risk of SIDS. The hypoxic ventilatory response is characterized by eupneic augmentation and sigh generation followed by a state of gasping (15). Impaired arousal and autoresuscitation during these periods are generally the focus of SIDS research. However, a third and potentially critical, yet often overlooked phase of respiratory vulnerability occurs upon reoxygenation. During reoxygenation, a silent period termed the post-hypoxic ventilatory depression creates a transition between gasping and eupnea (16). Any impairment that increases the length of the post-hypoxic ventilatory depression or otherwise impairs the reestablishment of eupnea may contribute to SIDS. This may be especially true when early resuscitation efforts fail during SIDS events. Sleep-related Causes of SUID and SIDSThere is no evidence that aspiration is more common among healthy infants who sleep in the supine position than among healthy infants who sleep in the prone position (17). Furthermore, in countries (including the United States) that have seen a major change in infant sleep position – from mainly stomach sleeping to mostly back sleeping – the incidence of serious fetal chocking has not increased (17). In fact, babies may actually clear secretions better when placed on their backs. When babies are in the back-sleep position, the trachea lies on top of the esophagus (see figure no. 1 below). Anything regurgitated or refluxed from the esophagus must work against gravity to be aspirated into the trachea. Conversely, then an infant is in the stomach sleep position, anything regurgitated will pool at the opening of the trachea, making it easier for the infant to aspirate (see figure no. 2 below). Also, chemo-sensitive tissue that initiates the reflex is more prominent on the posterior versus anterior pharyngeal wall, thus suggesting an even greater protection against aspiration when the baby is lying on his or her back. 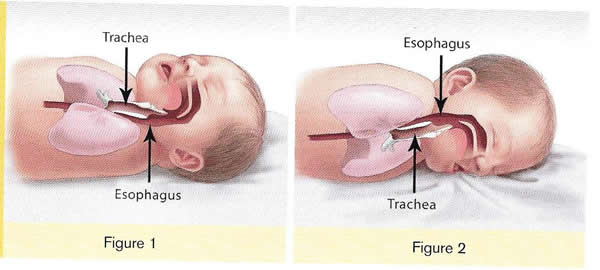 1. Here the trachea (airway is above the esophagus (food pipe). Anything that is spit up will be pushed back down by gravity to the lowest point. The airway is protected. 2. On the stomach, the trachea (airway) is below the esophagus (food pipe). Anything that is spit up will be pushed back down by gravity to the lowest point. On the stomach it is easier to aspirate into the lungs. Source: U.S. Department of Health and Human Services Of the very few reported cases of death due to chocking, most of the infants were in the stomach sleep position. Healthy babies should always be placed on their backs to sleep for naps and at night. Babies with certain upper-airway malformations (e.g. Robin syndrome) may have acute airway obstructive episodes that are relieved by prone positioning (19). However, these cases are rare; health care providers should clearly state the reasons for the prone recommendation to the parents and caregivers in these cases. Current evidence suggests that even infants with gastroesophageal reflex should be placed on their backs to sleep, with the rare exception of infants for whom the risk of death from gastroesophageal reflux is greater than the risk of SIDS (19). There may be other infants for whom the risk/benefit balance favors stomach sleeping. Health care providers should consider the potential benefit to the infant when recommending sleep position. If medical personnel determines that the stomach sleep position is necessary because of a medical condition or other concern, health care providers should advise parents and caregivers to reduce the risk of SIDS in other ways, such as by avoiding soft bedding and ensuring that infant do not overheat during sleep (20). For most infants, however, stomach and side sleeping are not advised. Preterm Infants and Sleep PositionResearch show that preterm infants are at higher risk for SIDS simply because they were born preterm, defined as before 37 weeks' gestation; therefore, placing preterm infants on their backs for sleep is a critically important way to reduce the risk of SIDS (21). Preterm infants who have active respiratory disease may have improved oxygenation if they are placed on their stomachs. Thus, the stomach sleep position during acute respiratory disease may be appropriate for infants in a highly monitored, inpatient setting. Because preterm babies often remain in the hospital for several days to weeks before discharge, these infants be placed on their backs to sleep as soon as possible after the respiratory condition has stabilized. This practice will allow parents and caregivers to become familiar with the position they should use at home. Providers should clearly state and strongly recommend that parents and caregivers be especially diligent about making sure their infants are placed in the back-sleep position for every sleep time to reduce the risk of SIDS. Epidemiological studies have shown that, when placed on their stomachs to sleep at home, low birth weight or preterm babies may be at higher risk for SIDS than babies born at or after 37 weeks' gestational age (22). The prone sleeping position – an exogenous stressor of respiratory physiology in SIDS In 1985, Davies described the exceedingly rare occurrence of cot deaths (i.e. SIDS) within the Hong Kong population when compared to the occurrence in western countries, and while several possible reasons were proposed for this difference, Davies recognized the potential that infant sleep position may have in SIDS. Subsequent reports found epidemiological and clinical evidence to support that the prone sleeping position correlates with increased risk of SIDS (23). As an exogenous stressor of SIDS, a wide variety of events may occur leading to infant death when in the prone sleeping position. These events range from thermal stress, to airway compression or collapse, to re-breathing of exhaled gases (24). However, a common intersection among these events appears to be the occurrence of a catastrophic life-threatening event involving cardio-respiratory distress that the SIDS victims does not successfully overcome. It is important, however, to recognize that while the prone sleeping is a risk factor alone, it does not cause SIDS. SIDS occurs only when environmental challenges (i.e. extrinsic risk factors) and intrinsic risk factors intersect. Thus, while the prone sleeping position may set the stage for SIDS, only vulnerable infants succumb to SIDS. The final common pathway for multiple risk factors leading to SIDS The final common pathway for SIDS involves a failure to arouse and autoresuscitate in response to environmental challenge. Many intrinsic and extrinsic factors increase SIDS susceptibility including gender, genetic polymorphisms, prenatal nicotine exposure, and temperature. These risk factors can directly alter the function of the preBÖtC and impair the ability of this network to generate sigh and gasping rhythm, as well as coordinate cardiorespiratory coupling between the Risk Factors for SUID and SIDSMore and more research evidence suggests that infants who die from SIDS are born with brain abnormalities or defects. These defects are typically found within a network of nerve cells that rely on a chemical called serotonin that allows one nerve cell to send a signal to another nerve cell. The cells are located in the part of the brain that probably controls breathing, health rate, blood pressure, temperature, and waking from sleep. But scientists believe that brain defects alone may not be enough to cause a SIDS death. Evidence suggests that other events must also occur for an infant to die from SIDS. Researchers use the Triple-Risk Model to explain this concept. In this model all three factors have to occur for an infant to die from SIDS (See figure 4 below). Having only one of these factors may not be enough to cause death from SIDS, but when all three combine, the chances of SIDS are high. 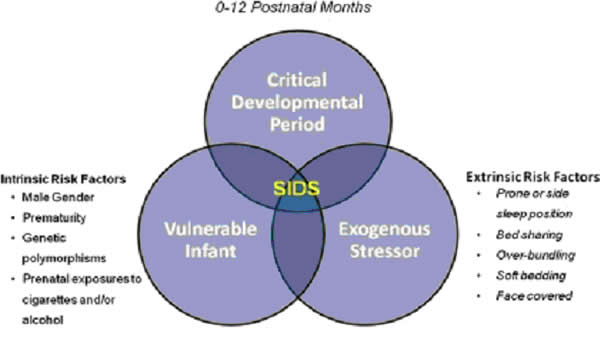 These factors are (25):
According to the Triple-Risk Model, all three things have to be present for SIDS to occur. How to reduce risk of Sleep-related infant death (26):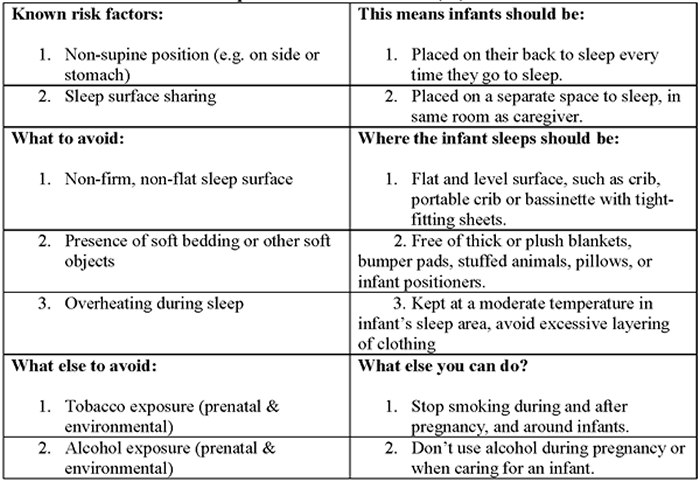 Safe To Sleep CampaignThe Safe to Sleep ® campaign, formerly known as the Back to Sleep campaign, has helped educate millions of caregivers, parents, grandparents, aunts, uncles, babysitters, childcare providers, health care providers, and others, about ways to reduce the risk to reduce SIDS and other sleep-related causes of infant death (27). Today, the Safe to Sleep campaign builds on the successes of Back to Sleep to address SIDS and other sleep-related causes of infant death and to Healthy Native Babies Project (HNBP) Data show that SUID and SIDS are disproportionately higher in American Indian/Alaska Native communities. From 2011 through 2014, the overall SUID rate per 100,000 live birth for American Indian/Alaska Native infants (194.1) was more than twice those of non-Hispanic white infants (83.8). SIDS deaths account for the largest proportion of SUIDs for all racial and ethnic groups including American Indian/Alaska Native communities. National Institute of Child Health and Human Development (NICHD) working with representatives from Tribes in the Northern Tier and others who serve American Indian/Alaska Native audiences – launched HNBP in 2003 to assist programs in addressing safe infant sleep in these communities. As the HNBP enters its 15th year, NICD, working group members, and other partners are reexamining the approach and the resources to better meet the changing needs of American Indian/Alaska Native communities (28). Ways to Help Your Baby Sleep SafelyResearch shows that there are several ways to reduce the risk of SIDS and other sleep-related causes of infant deaths. Safe sleep environment is summarized below:
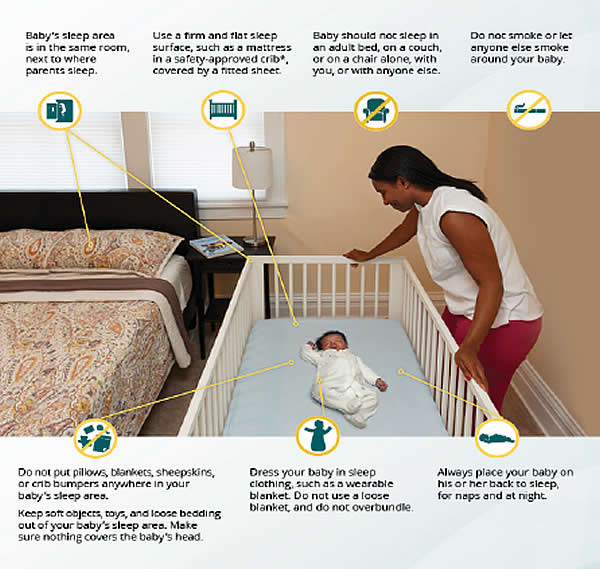 Parents and caregivers are frequently concerned about the appropriate strategy for infants who have learned to roll over, which generally occurs at 4 to 6 months of age. As infants mature, it is more likely that they will roll. In one study, 6% to 12% of 16 to 23 week-old infants placed on their backs or sides, respectively, were found in the prone position; among infants aged 24 weeks or older, 14% of those placed on their sides were found in the prone position (29). Repositioning the sleeping infant to the supine position can be disruptive and might discourage the use of supine position altogether. Although data to make specific recommendations as to when it is safe for infants to sleep in the prone position is lacking, the American Academy of Pediatrics (AAP) recommends that these infants continue to be place supine until 1 year of age. If the infant can roll from supine to prone and from prone to supine, the infant can then be allowed to remain in the sleep position that he or she assumes. To prevent suffocation or entrapment if the infant rolls, soft or loose bedding should continue to be removed from the infant's sleep environment. Some caregivers use such bedding to prevent infant from rolling, but this bedding could cause suffocation and entrapment. Parents can be reassured by the information that the incidence of SIDS begins to decline after 4 months of age. Tummy TimeTummy Time describes the times when you place your baby on his or her stomach while your baby is awake and while someone is watching. Tummy Time is important because it:
 Supervised, awake Tummy Time on a daily basis can promote motor development and minimize the risk of optional plagiocephaly. Position plagiocephaly or plagiocephaly without synostosis, can be associated with supine sleeping position (30). It is most likely to result if the infant's head position is not varied when placed for sleep, if the infant spends little or no time in awake, supervised tummy time, and if the infant is not held in the upright position when not sleeping. Children with developmental delay and/or neurologic injury have increased rates of plagiocephaly without synostosis, although a causal relationship has not been demonstrated. In healthy normal children, the incidence of plagiocephaly without synostosis decreases spontaneously from 20% at 8 months to 3% at 24 months of age (30). Although data to make specific recommendations as to how often and how long tummy time should be undertaken are lacking, supervised tummy time while infant is awake is recommended on a daily basis. Tummy time should begin as early as possible to promote motor development, facilitate development of the upper body muscles, and minimize the risk of positional plagiocephaly. From the day they come home, babies benefit from 2 to 3 Tummy Time sessions each day for a short period of time (3 to 5 minutes). As the baby grows and shows enjoyment of Tummy Time, you can lengthen the sessions. As babies grow older, more Tummy Time helps build strength for sitting up, rolling over, crawling, and walking. These suggestions can help you and your baby enjoy Tummy Time:
Ways to help prevent flat spots on your baby's head
Home Heart and Breathing Monitors and SIDSThere is no evidence that apparent life-threatening events are precursors to SIDS, and infant home monitors should not be used as a strategy for preventing SIDS (31). For many years it was believed that apparent life-threatening events were the predecessors of SIDS, and home apnea monitors were used as a strategy for preventing SIDS. However, there is no evidence that home monitors are effective for this purpose. The Task Force concurs with the American Academy of Pediatrics (AAP) Committee on Fetus and Newborn, which has recommended that infant home monitoring not be used as a strategy to prevent SIDS, although it can be useful for some infants who have had an apparent life-threatening event (31). Many SUID and SIDS parents ask about the use of a home apnea-bradycardia monitor to alert the caregiver should a life-threatening event occur, and/or to reduce parental anxiety for siblings. Three is no scientific evidence that home apnea-bradycardia monitoring prevents infant or child deaths, and siblings, some physicians and parents may wish to use home apnea bradycardia monitors for diagnostic reasons, and/or emotional reassurance. In these instances, the monitors need not be used after 3-6 months of age unless abnormalities are found. Other types of monitors such as video, motion, sound and temperature are also available through most baby product stores. Ultimately, parents considering the use of monitors for their SIDS sibling should discuss all these issues with their pediatrician, who can help them make a better choice based on their specific situation and needs. Vaccines (Immunizations) and SIDSInfants should be immunized in accordance with recommendations of the AAP and CDC. Vaccines have not been shown to cause SUID or SIDS. Babies receive multiple vaccines when they are between 2 to 4 months old. This age range is also the peak age for SIDS. The timing of the 2-month and 4-month shots and SIDS has led some people to question whether they might be related. However, studies have found that vaccines do not cause and are not linked to SIDS. Multiple research studies and safety reviews have looked at possible links between vaccines and SIDS. The evidence accumulated over many years do not show any links between childhood immunization and SIDS (32). Centers of Disease Control and Prevention (CDC) and Food and Drug Administration (FDA) are committed to ensuring that vaccines provided to the public are safe and effective. Once vaccines are licensed in the United States, CDC and FDA continuously monitor them through several safety systems (33). CDC monitors the safety of vaccines by:
Breastfeeding and SIDSBreastfeeding is recommended. Earlier epidemiologic studies were not consistent in demonstrating a protective effect of breastfeeding on SIDS; some studies found a protective effect and others did not (34). Because many of the case-control studies demonstrated a protective effect of breastfeeding against SIDS is univariate analysis but not when confounding factors were considered, these results suggested that factors associated with breastfeeding, rather than breastfeeding itself, are protective. However, newer published reports support the protective role of breastfeeding on SIDS when considering potential confounding factors. Currently in the United States, 73% of mothers initiate breastfeeding, and 42% and 21% are still breastfeeding at 6 and 12 months, respectively. Non-Hispanic black mothers are least likely to initiate or to still be breastfeeding at 6 and 12 months (54%, 27%, and 12%, respectively), whereas Asian/Pacific Islander mothers initiate and continue breastfeeding more than other groups 81%, 52%, and 30%, respectively). Rates for initiating and continuing breastfeeding at 6 and 12 months for non-Hispanic white mothers are 74%, 43%, and 21%; rates for Hispanic mothers are 80%, 45%, and 24%; and rates for American Indian/Alaskan Native mothers are 70%, 37% and 19%, respectively (35). Physiologic sleep studies have found that breastfed infants are more easily aroused from sleep than their formula-fed counterparts. In addition, breastfeeding results in a decreased incidence of diarrhea, upper and lower respiratory infections, and other infectious diseases that are associated with an increased vulnerability to SIDS and provides overall immune system benefits from maternal antibodies and micronutrients in human milk. Exclusive breastfeeding for 6 months has been found to be more protective against infectious diseases compared with exclusive breastfeeding to 4 months of age and partial breastfeeding thereafter. If a breastfeeding mother brings the infant into the adult bed for nursing, the infant should be returned to a separate sleep surface when the mother is ready to sleep. Although bed-sharing may facilitate breastfeeding, it is not essential for successful breastfeeding. Furthermore, studies have found that the risk of SIDS while bed-sharing was similar regardless of breastfeeding status, which indicates that the benefits of breastfeeding do not outweigh the increased risk associated with bed-sharing. Pacifier UseConsider offering a pacifier at nap time and bedtime. Several studies have found a protective effect of pacifiers on the incidence of SIDS, particularly when used at the time of last sleep. Two meta-analyses revealed that pacifier use decreased the risks of SIDS by 50% to 60% (36). The mechanism of this apparent strong protective effect is still unclear, but lowered arousal thresholds, favorable modifications of autonomic control during sleep, and maintaining airway patency during sleep have been proposed. It is common for the pacifier to fall from the mouth soon after the infant falls sleep; even so, the protective effect persists throughout that sleep period. The AAP policy statement on breastfeeding and the use of human milk includes a recommendation that pacifiers can be used during breastfeeding, but implementation should be delayed until breastfeeding is well established. Genetic Factors and SIDSGenetic studies in SIDS have been motivated by clinical, epidemiological, and/or neuropathological observations in SIDS victims, with subsequent pursuit of candidate genes in five categories (37):
A number of genetically controlled pathways appear to be involved in at least some cases of SIDS. Given the diversity of results to date, genetic studies support the clinical impression that SIDS is heterogeneous with more than one entity and with more than one possible genetic etiology. Future studies should consider expanded phenotypic features that might help clarify the heterogeneity and improve the predictive value of the identified genetic factors. Such features should be evaluated to the extent possible in both SIDS victims and their family members. Newborn Care and Safety: Recommendations "Suffocation Prevention"Attendance of prenatal classes varies among patient populations and tends to be low in women of limited means and of young age, leaving the obstetric care team to be the primary source of information. Given the shortening length of hospital stays and the exhaustion most women experience surrounding childbirth, there is little time for in-depth newborn safety education. Many authors have suggested newborn care and safety education should be provided at multiple times during pregnancy including the antepartum period (38). In 2016, AAP's recommendations to reduce the risk of SIDS and other sleep related infant deaths are based on epidemiologic studies that include infants up to 1 year of age (39). Therefore, recommendations for sleep position and the sleep environment, unless otherwise specified, are for the first year after birth. Healthcare providers are encouraged to have open and non-judgmental conversations with the families about their sleep practices. Individual medical conditions may warrant that a healthcare provider make different recommendations after weighing the relative risks and benefits. Infant Loss SupportThe loss of a child is tragic for all involved. Grief is immense and can be all-consuming. A sudden unexplained death offers even more unanswered questions for both families and their physicians. "Will this happen again? If no one can tell me why my child died, how can anyone be sure it won't happen again? How can I be proactive to protect my family, and have some peace of mind?" Families who are coping with a sudden unexplained death of a family member often have anxiety about their family health and the risk of recurrence. Grief is the emotional response to the death of a loved one. It is a normal consequence of profound interpersonal loss yet involves extreme cognitive and emotional symptoms in most bereaved individuals. A significant minority of bereaved individuals experience a more enduring grief response that exceeds social norms and causes impairment in daily functioning. Prolonged grief disorder (PGD), formerly call complicated grief and alternatively called persistent complex bereavement disorder, is diagnosed as a pathologic response at the extreme of grief and is distinct from posttraumatic stress disorder or depression. PGD involves the persistence of separation distress, characterized by significant emotional pain and yearning in addition to cognitive, emotional, and behavioral symptoms more than 6 months after a significant loss. A recent meta-analysis found PGD in 9.8% of bereaved persons 1 year after loss (40). In this study half of the mothers suffered from PGD in the 4 years after their infants' deaths from SIDS, with daily, intrusive emotional pain and yearning in 68.1% (41). Their specific grief-related symptoms may be potential targets for screening and referral. Severe symptoms and heightened risk for PGD is seen in mothers after their infants died of SIDS, with discernable symptom profiles. Given the involvement of pediatricians with the families after SIDS, they may have a unique role in identifying this problem and helping address its consequences and referrals to the specialist when needed. SummaryCertain causes of newborn mortality such as sudden unexplained infant death, which includes sleep-related infant death and sudden infant death syndrome, are potentially preventable. Obstetricians are uniquely positioned to counsel new parents about the practices regarding newborn sleep, feeding, and transportation. Newborn safety should be routinely taught in obstetrics curricula, and the Women's Health and Education Center (WHEC) has partnered with the United Nations (UN) and the World Health Organization (WHO), to disseminate updated literature and guidelines to health care providers regarding newborn safety. Several critical but avoidable risk factors for SIDS have been identified, dramatically reducing SIDS incidence. However, SIDS remains a serious problem underscoring the need for a better understanding of the underlying causes. Anatomical evidence indicates SIDS is associated with specific pathological abnormalities in the medulla involving neuromodulators known to be involved in the neuronal control of breathing. Hence, the next step is to unravel how these pathologies and risk factors combine to contribute to a child's death. By expanding the network of clinicians, scientists, and families working together, and by combined efforts in a collaborative multi-center study of candidate genes and/or genomics, the discovery of the genetic profile of the infant at risk for SIDS can ultimately be determined. References
|