Anemia por deficiencia de hierro en el embarazo
WHEC Boletín Práctica Clínica y de gestión para proveedores de atención médica. Educación subvención prevista de Salud de la Mujer y el Centro de Educación (WHEC).
Iron deficiency is magnified in pregnancy because the developing fetus demands 50% of mother’s iron endowment. Approximately 50% of first-trimester, non-anemic pregnant patients have iron deficiency based on levels of serum ferritin, transferrin saturation, or both (1). Prospective neonatology evidence shows that 25% of infants born to mothers with iron deficiency are depleted to a level associated with increased developmental, cognitive, and behavioral deficits. However, although oral replacement improves mothers’ hematologic parameters, neonatal ferritin levels remain depleted. The high level of iron deficiency in the first trimester suggests that the frequent finding of iron deficiency anemia during pregnancy may be related to iron deficiency before pregnancy. Although iron deficiency may be in part secondary to deficient nutrition, the more likely culprit is heavy menstrual bleeding draining iron reserves before pregnancy even begins. Regardless, and unlike initiatives for normalization of folate and glucose levels before pregnancy, no United State (U.S.) guidance suggests that individuals contemplating pregnancy should have hemoglobin measured, it would seem prudent, to revisit current paradigm that ignores iron status in reproductive-aged females outside of pregnancy, not only to reduce the need for oral or intravenous (IV) iron during and after pregnancy but perhaps to ultimately improve fetal development, neonatal iron stores, and outcomes.
The purpose of this document is to review the current management of iron deficiency anemia in pregnancy and the current policy of oral and IV iron replacement therapy, during pregnancy and postpartum. New evidence suggests that intermittent dosing is as effective as daily or twice-daily dosing with fewer side effects. For patients with iron deficiency anemia who cannot tolerate, cannot absorb, or do not respond to oral iron, intravenous (IV) iron is preferred. With contemporary formulations, allergic reactions are rare.
Prevalence
Today, iron is the world’s most common micronutrient deficiency, estimated to affect 3 billion people and more than half of pregnancies worldwide, with higher prevalence in under-resourced countries (2). There are no countries for which country-level estimates were generated where anemia is not at least a mild public health problem (i.e. prevalence is at least 5%) in children and women. Available meta-analysis suggest that iron supplementation would increase the mean blood hemoglobin concentration by 8.0 g/L in children, 10.2 g/L in pregnant women and 8.6 g/L in non-pregnant women. Applying these shifts to estimate blood hemoglobin concentrations indicates that about 42% of anemia in children would be amenable to iron supplementation and about 50% or anemia in women could be eliminated by iron supplementation.
Reducing anemia is recognized as an important component of the health of women and children, and the second global nutrition target for 2025 calls for a 50% in women of reproductive age (2). In low-income countries, the prevalence of anemia remains high and is an area of priority. If the current trends are maintained, there is probability of less than 25% in all regions of reaching the global target of reducing the prevalence of anemia by 50% in women of reproductive age. To make a significant impact, it is likely that a combination of key programs that address the determinants of low blood hemoglobin concentrations will be required. These strategies should be tailored to local conditions, taking into account the specific etiology and prevalence of anemia in a given setting and population group, and should be built into the primary healthcare systems and existing programs.
Definitions
Anemia is defined as a low red blood cell (RBC) count, a low hematocrit (the proportion of blood volume that is RBCs), or a low hemoglobin concentration (the oxygen carrying protein of RBCs). Hemoglobin concentration is measured in grams per liter or grams per deciliter. In pregnancy, a hemoglobin concentration of less than 11.0 g/dL in the first trimester and less than 10.5 g/dL, in the second and third trimester is considered anemia (3). There are various guidelines of anemia (see table 1).
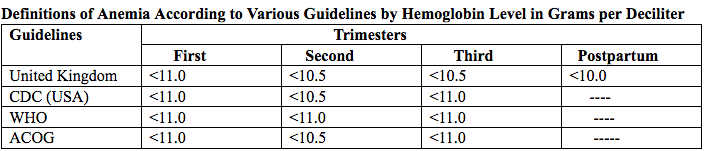
Table 1. CDC: Centers for Disease Control and Prevention (in the United States); WHO: World Health Organization; ACOG: American College of Obstetricians and Gynecologists.
Hematological and biochemical markers of anemia
Measuring hemoglobin levels alone may not reliably indicate anemia, because of the extensive overlap in hemoglobin values between healthy and anemic people (4). Mean corpuscular volume (MCV), red cell distribution width (RDW), reticulocyte count, serum ferritin, and serum transferrin receptor (TfR) levels are other measurements that can help clinicians evaluate anemia. Although they are not definitive, these measurements often clues to the underlying causes of anemia.
Mean corpuscular volume (MCV): normally 90 fL ± 9 fL, can accurately detect increases (macrocytosis) in the size of RBCs.
Red cell distribution width (RDW): is an index of the distribution of red cell volumes and a mathematical representation of variations in the size of red blood cells.
Reticulocyte count: measures the number of newly released RBCs. A high reticulocyte count indicates that the anemia is probably caused by a loss of erythrocytes and subsequent release of new cells as the bone-marrow compensates for the erythrocyte loss. Conversely, a low reticulocyte count indicates a low rate of production of new erythrocytes. The reticulocyte count normally ranges from 0.5% to 1.7% of whole blood.
Serum ferritin: measurements provide and easily obtained, fairly accurate measure of body’s iron stores (5). Iron is stored as ferritin and hemosiderin. When iron availability in a cell is high, ferritin production is promoted. Under normal conditions, serum ferritin is in equilibrium with the ferritin contained in tissue iron stores: 1 ng/mL of serum ferritin corresponds to approximately 8 mg of stored iron. The average value of serum ferritin in adult women younger than 40 years is 12 to 122 ng/mL and women older than 40 years is 12 to 250 ng/mL. The drawback to measuring serum ferritin is that values may be elevated independently of iron stores if the patient has inflammation, malignancy, or liver disease.
Transferrin receptor (TfR): levels above 8.5 mg/L indicate depleted iron stores. TfRs are found on all cells that require large amounts of iron such as placental cells, certain tumor cells, and erythroid precursors in the bone marrow. When iron stores are depleted, erythroid precursors generate and release more transferrin receptors into the blood. This marker is especially useful for assessing anemia in pregnant women and is not influenced by the presence of inflammation.
Serum transferrin saturation: another clinical marker, is calculated as the ratio of serum iron to total-binding capacity. Transferrin saturation is a useful clinical indicator of how much iron is available for erythropoiesis.
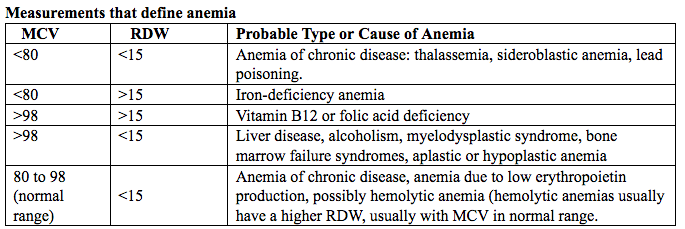
Table 2. MCV: mean corpuscular volume; RDW: red cell distribution width.
Assessing Anemia
What do the markers indicate?
The diagnostic characteristics of iron deficiency anemia are different from those of anemia due to chronic disease. With iron deficiency anemia, iron is low and blood TfR levels, indicating high total iron-binding capacity, are high; the transferrin saturation and ferritin levels, however, are low. In anemia of chronic disease, serum iron and total iron-binding capacity are both low; transferrin saturation is usually low; ferritin levels are normal or increased; and TfR levels are normal (6).
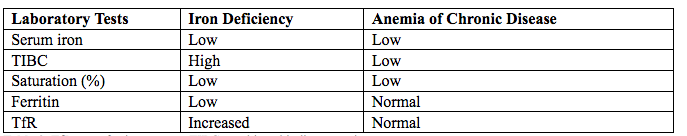
Table 3. TfR: transferrin receptor; TIBC: total iron-binding capacity.
Iron Transport and Storage
Most of the body’s iron resides in RBCs, which have an iron content of 31 mg per kilogram of body weight. Muscle iron, or myoglobin comprises 4 mg per kilogram of body weight. A small but important amount of iron is also contained in metallo-flavoprotein enzymes and other enzymes (1mg/kg). The remainder of the body’s iron is stored in the liver, which may contain from 0 mg/kg (iron deficiency) to 20 mg/kg (borderline overload).
Iron transport is cycling continuously (see figure 1). It can be viewed as beginning with new RBC production in the bone marrow. After leaving the bone marrow, RBCs normally circulate for 120 days, after which they are taken up into macrophages throughout the body and especially in spleen. Senescent RBCs are broken down to release iron back into the bloodstream, which is transported by transferrin and returned to the bone marrow. This recycling process provides most of the iron required for erythropoiesis. A small portion of the iron does not return to the marrow but is stored in the liver. The body normally absorbs and loses 1 to 2 mg of iron/day and maintains a steady iron level by adjusting the amount of iron absorbed from the diet (7).
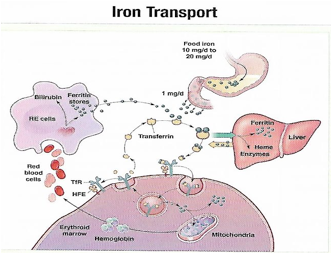
Figure 1. HFE: human hemochromatosis protein (enzymes); RE: reticulum; TfR: transferrin receptor.
In anemic states, less iron travels back through the pathway from the macrophages to the bone marrow, and the additional iron needed to make RBCs must be obtained either from iron stores or absorbed from the gastrointestinal (GI) tract (8). An increase in erythropoiesis drive, such as that experienced by patients treated with erythropoietin for anemia caused by chronic renal failure or chemotherapy, also depletes iron stores and increases absorption of iron from the GI tract.
A small amount of iron is excreted each day. Men and non-menstruating women lose approximately 1 mg or iron daily in feces from desquamated mucosal cells and skin cells. This loss is readily replaced by most diets. As the result of menstrual blood loss, menstruating women are required to absorb approximately 1.5 to 2 mg iron per day. Blood loss through excessive menses is the primary cause of anemia in pre-menopausal women.
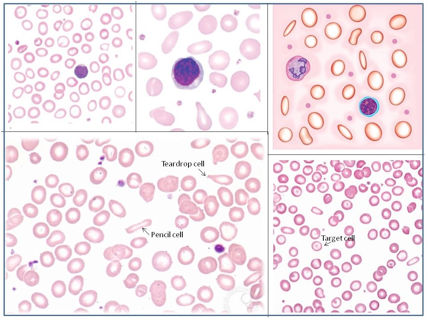
Figure 2. Laboratory diagnosis of iron deficiency anemia. Peripheral blood smear.
Red Blood Cells (RBCs)
Microcytosis - RBCs are usually smaller than normal. Dimorphic blood picture is seen with a dual population of red cells of which one is macrocytic and the other microcytic and hypochromic when iron deficiency is associated with severe folate or vitamin B12 deficiency.
Hypochromasia - central pallor in RBCs is more than 1/3.
Poikilocytosis - Elliptical forms are common, and elongated pencil (cigar) shaped cells may be seen. Target cells and teardrop cells may also be present in small numbers. Severe anemia show ring/pessary cells.
Screening for Anemia in Pregnancy
Although the U.S. Preventive Services Task Force does not recommend routine screening for anemia in pregnancy, a complete blood count (CBC) is typically obtained at registration for prenatal care, again at the start of third trimester, on admission for labor and delivery and possibly postpartum depending on blood loss and mode of delivery. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on anemia in pregnancy recommends screening for anemia with a CBC in the first trimester and again at 24 0/7 to 28 6/7 weeks of gestation (9). The components that are of interest in screening for anemia are the hemoglobin, hematocrit and mean corpuscular volume (MCV). If anemia is present, the MCV is useful in classifying the anemia.
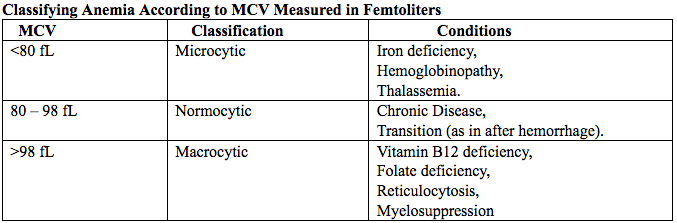
Table 4. MCV: mean corpuscular volume.
Physiological Changes in Blood Volume during Pregnancy
An important contributor to anemia in pregnancy, no matter what other causes of anemia may be present, is the physiological changes of pregnancy. In 1934, Dieckmann and Wegner published a systematic study of serial blood measurements throughout the pregnancy and the puerperium. Although suspected earlier, they were able to confirm that, although plasma volume, red cell mass, and hemoglobin levels increase throughout pregnancy, the increase in plasma volume is disproportionately greater than the increase in red cell mass or hemoglobin and results in what they described as an “oligocythemic hypervolemia.” In general, blood volume increases during pregnancy by 30 - 50% above baseline (10). These hemodilutional changes serve to reduce the viscosity of maternal blood, and in theory, improve uteroplacental perfusion.
Maternal Consequences of Anemia
The maternal consequences of anemia may manifest during pregnancy, at the time of delivery or during the postpartum period, and may or may not include signs and symptoms consistent with a low level of circulating RBCs. Signs and symptoms, depending on the severity of the anemia, the rapidity of its onset, and the presence of other underlying conditions, may include fatigue, pallor, light-headedness, tachycardia, dyspnea, poor exercise tolerance, suboptimal work performance and depressed mood (11). Maternal anemia is associated with significantly increased risk of cesarean delivery, postpartum anemia, and blood transfusion (11).
Confirmation of the Diagnosis of Iron Deficiency Anemia
In the case of anemia, the gold standard for the diagnosis of iron-deficiency has become a low ferritin level (12). Ferritin is a hollow, globular intracellular protein that has the capacity, by crystallization with phosphate and hydroxide ions, to store up to 4,500 ferric (F+3) ions and release them, as necessary. Therefore, serum ferritin is released into circulation where ferritin serves as an iron carrier. Therefore, serum ferritin serves as an indirect measure of the total iron body stores. Serum iron is a measure of the total amount of circulating iron that is bound to either ferritin (10%) or transferrin (90%). Transferrin is a glycoprotein that can bind two ferric ions and transport iron through the body to various tissues including the liver, spleen and bone marrow. Total iron binding capacity is a measurement of amount of transferrin’s available binding sites (13). Transferrin saturation is the ratio of serum iron to the total iron binding capacity divided by100, which represents the percentage of transferrin’s iron-binding sites being occupied by iron. A low transferrin saturation is indicative of iron deficiency and can be used in the diagnosis of iron deficiency when iron deficiency is suspected, but ferritin is normal. When ferritin is low, no other laboratory testing may be necessary.
Evaluation of Iron Deficiency Anemia
Mild Anemia; Hemoglobin 10.0 g/dL or higher
Mean capsular volume (MCV):
If normocytic or mildly microcytic, a trial of oral iron can be diagnostic and therapeutic;
If very microcytic (MCV less than 75)), suspect abnormal hemoglobin and obtain hemoglobin electrophoresis;
Folate level and vitamin B12 Levels: If either is low, treat accordingly; If neither is low, refer to hematology for further evaluation.
Moderate Anemia; Hemoglobin 7.0 - 9.9 g/dL
Hemoglobin electrophoresis:
If not performed already
Folate level and Vitamin B12 levels:
If either is low, treat accordingly;
If neither is low, refer to hematology.
Serum ferritin:
If less than 30 ng/dL, presume iron deficiency and treat accordingly.
Serum creatinine:
To evaluate renal function.
Reticulocyte count:
To ensure bone marrow activity.
Serum iron:
To help confirm a diagnosis of iron deficiency anemia.
Total iron binding capacity:
Elevated in pregnancy and iron deficiency anemia.
Transferrin saturation:
If less than 15-20%, iron deficiency anemia is confirmed.
Severe Anemia; Hemoglobin 4.0-6.9 g/dL
Obtain the laboratory studies, stated above, start treatment for nutrient deficiency if present, and refer to hematology for further evaluation.
Fetal, Neonatal, and Childhood Consequences of Maternal Anemia
Many studies have reported that maternal anemia is associated with adverse fetal, neonatal and childhood outcomes such as perinatal mortality, neonatal mortality, low-birth weight, pre-term labor and birth, and small-for-gestational age newborns (14). Maternal anemia has also been associated with behavioral and neurodevelopmental abnormalities (15). Although these systematic reviews and meta-analyses found an association between maternal anemia and some adverse fetal and neonatal outcomes, a causal relationship has not been established. Anemia may be a contributor to adverse fetal and neonatal outcomes or may merely accompany these conditions that negatively affect fetal and neonatal well-being. At a minimum, maternal iron deficiency anemia is correlated with lower cord-blood serum ferritin levels (16). In theory, if iron deficiency anemia causes such adverse outcomes, iron supplementation should reduce the risk of behavioral and neurodevelopmental abnormalities. One randomized trial, which examined the outcomes of IQ (intelligent quotient) and behavior for 4 years of age, did not find a benefit of iron supplementation (17). Other studies, however, as well as assessments other than IQ and behavior, would be required to support or refute the benefit of iron supplementation. Based on a systematic review of randomized trials commissioned by the United States Agency for Health Care Quality and Research in preparation for updating the U.S. Preventive Services Task Force recommendations regarding iron deficiency anemia in pregnant women, there is limited evidence that iron supplementation has any effect on fetal, neonatal or childhood outcomes (18).
MANAGEMENT
Prevention of Iron Deficiency Anemia
Primary prevention of iron deficiency anemia includes adequate dietary intake of iron and a low dose iron supplementation averaging about 30 mg/day. Education about adequate dietary intake of iron as well as folate and vitamin B12, from the dietary sources of these nutrient, is important.
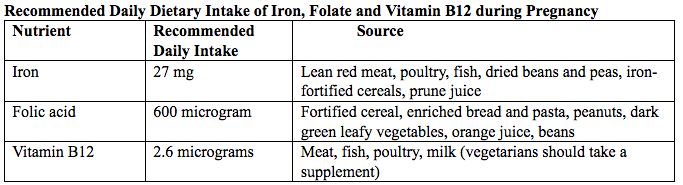
Table 5. Source: American College of Obstetricians and Gynecologists. Nutrition during pregnancy (19). 70% of pregnant women in the United States take a prenatal vitamin with iron or take an additional iron supplement. Iron content ranges from 9 to 60 mg (mean 26.3 mg) per dose of non-prescription prenatal vitamins and 4.5 to 106 (mean 34.7 mg) per dose of the prescription prenatal vitamins. The U.S. Preventive Task Force found insufficient evidence that routine iron supplement in non-anemic pregnant women improves clinical outcomes for women or their children (20), but they did find evidence that routine iron supplementation may improve maternal hematologic indices.
First-Line of Treatment
The first-line treatment of iron deficiency anemia is oral iron. In iron deficiency anemia, treatment is considered necessary and effective. Historically, treatment has consisted of a twice-daily dose of an oral formulation with ferrous sulfate 325 mg (65 mg of elemental iron), in addition to the low dose of iron contained in a prenatal vitamin as well as dietary iron (21). Vitamin C is often prescribed along with oral iron to improve absorption; although there is no clinical evidence that supplemental vitamin C actually improves iron absorption. Absorption of iron from oral supplements may be improved when taken between meals or at bedtime, but there are few data. One study found that serum ferritin levels were slightly, but statistically significantly, higher in patients who took oral iron supplements between meals or at bedtime, rather than with meals (22).
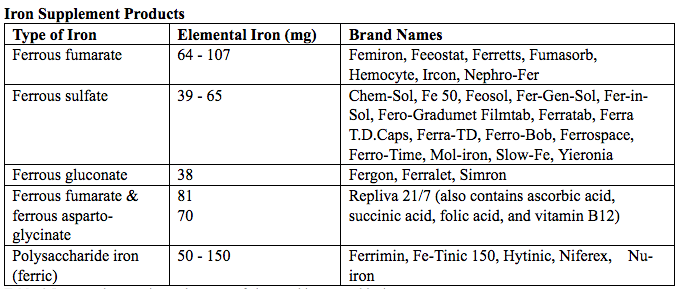
Table 6. Iron supplementation and amount of elemental iron per tablet in mg.
Potential side effects: therapeutic doses of iron supplements may cause gastrointestinal side effects such as nausea, vomiting, constipation, diarrhea, dark-colored stools, and/or abdominal distress (23). Side effects may be minimized by starting with half the recommended dose and gradually increasing to the full dose. Iron from enteric-coated or delayed-release preparations may have fewer side effects, but the iron is not as well absorbed, and therefore is not usually recommended (23). Patients who cannot tolerate the tablet form of iron may be treated with ferrous sulfate solution. Treatment can begin with a dosage of 2 to 3 drops orally, gradually increasing to a standard dosage (24). Our current practice is to recommend oral iron be taken every other day, as opposed to daily or more than once a day.
Intravenous Iron
For patients with iron deficiency anemia who cannot tolerate, cannot absorb, or do not respond to oral iron, intravenous iron is preferred in the third trimester and sometimes as early as the second trimester. In general, both oral and intravenous iron raise hemoglobin and ferritin levels, but intravenous iron result in faster-rise and a greater increase in hemoglobin and ferritin than did oral iron (25). Gastrointestinal side effects are reported more often with oral iron, whereas a metallic taste, dizziness, hot flashes, and arthralgias are reported more frequently in intravenous iron group (26). Although these side effects are minor, the major drawbacks to intravenous iron are cost, logistics, and allergic reactions.
Intravenous iron formulations consist of an iron core and a carbohydrate shell that shows the release of iron and reduces its toxicity (27). The most serious allergic reaction is anaphylaxis. The highest risk is with high molecular weight iron dextran, which is no longer available (27). The risk of anaphylaxis with contemporary formulation is less than 1 per 1,000 administrations (28). The logistics of intravenous iron administration requires trained staff, pharmacy support, appropriate equipment and a designated space, typically an outpatient infusion center or a procedure room within a hospital. Before administration, the iron deficit was calculated using the formula (29):
Iron deficit (mg) = body weight (kg)X hemoglobin deficit (g/dL)X 2.4+(desired) depot iron (mg)
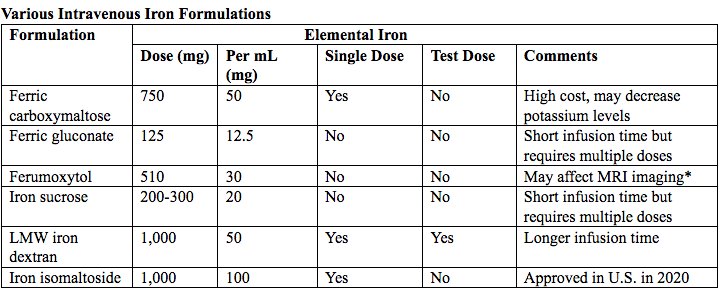
Table 7. MRI: magnetic resonance imaging; LMW: low-molecular weight.
*Ferumoxytol was originally developed as an MRI contrast agent. Its image-enhancing effects may be present for 72 hours or more after administration.
The most commonly used (92%) preparation in U.S. usually receive LMW (low-molecular weight) iron dextran. It can be administered in a single dose and is reliably covered by public insurances. Patient are usually pre-medicated prior or receiving LMW iron dextran with 25 mg of oral diphenhydramine and 650 mg of oral acetaminophen. Patients with significant asthma or multiple allergies also receive an additional 125 mg of intravenous methylprednisolone. A test dose of 25 mg of LMW iron dextran is administered over 15 minutes. Patients are observed for an additional 30 minutes for any allergic reaction before receiving the remaining dose of LMW iron dextran over 4 hours in 1L of normal saline.
Those who receive iron sucrose are premedicated with 25 mg or oral diphenhydramine and 650 mg of oral acetaminophen and receive their infusion over 2 hours. Those who receive ferumoxytol are not premedicated and receive their infusion over 15 minutes by slow intravenous push. Those who receive ferric gluconate are also not premedicated and receive their infusion over approximately 1 hour. A complete description of the program is published (30).
Transfusions of Red Cells in the Antepartum or Preoperative Period
Transfusions of red cells seldom are indicated unless hypovolemia from blood loss coexists or an operative delivery must be performed on a patient with anemia. The need for transfusion in women with antepartum complications can be predicted in only 24% of those who ultimately require blood products (9,31). The most common diagnoses associated with transfusion include trauma caused by instrumented delivery, uterine atony, placenta previa, retained products of conception, placental abruption, and coagulopathy (e.g., the syndrome of hemolysis, elevated liver enzymes, and low platelet count [HELLP]). The presence of these diagnoses in a patient with anemia should prompt consideration of transfusion, particularly in the presence of unstable vital signs (31).
Severe anemia with maternal hemoglobin levels less than 6 g/dL has been associated with abnormal fetal oxygenation, resulting in non-reassuring fetal heart patterns, reduced amniotic fluid volume, fetal cerebral vasodilation, and fetal death (32). Thus, maternal transfusion should be considered for fetal indications in cases of severe anemia. Autologous transfusions are rarely performed, and inability to predict the eventual need for transfusion has led to the conclusion that they are not cost-effective (9). Suggested criteria for consideration of autologous transfusion donation include an hematocrit greater than 32% at 32 weeks of gestation.
Erythropoietin
Erythropoietin is a hormone made in the interstitial fibroblasts of the kidney in response to hypoxia and anemia (33). It stimulates production of RBCs. During pregnancy, erythropoietin levels increase two to four times. In 1989, the U.S. Food and Drug Administration (FDA) approved the use of recombinant erythropoietin to treat anemia in dialysis patients (34). It has been used to treat iron deficiency anemia in pregnant patients with kidney disease, but has also been used as an adjunct to iron in the treatment of iron-deficiency anemia. Erythropoietin is a large molecule that does not cross the placenta, so fetal safety should not be a consideration. In pregnant patients who do not have kidney disease, however, the maternal safety of recombinant erythropoietin , when endogenous levels are already two to four times higher, is questionable due to possibility of an adverse event such as thromboembolism, hypertension or neurologic events. Recombinant erythropoietin is not approved for the treatment of iron deficiency anemia in pregnancy.
Postpartum Anemia
Postpartum anemia is a significant maternal co-morbidity that affects 50% of patients in the U.S. It has been associated with maternal impaired cognition, depression, and fatigue, ultimately affecting mother-child bonding and neonatal care. Oral iron supplementation is currently the first-line of treatment for women with iron-deficiency anemia in postpartum. The effectiveness of oral supplementation is diminished by variability in absorption, discomforting side effects, and poor compliance, limitations that can be overcome with intravenous iron. However, intravenous iron is costly and needs to be administered under supervision in a hospital or outpatient clinical setting. This study shows that it is feasible to administer intravenous iron, during the delivery admission, and the patients had higher levels of hemoglobin after 6 weeks postpartum, than those on oral iron (35). Intravenous iron given before discharge in the postpartum, while a patient is already in the hospital, may be more cost effective than oral iron. A larger multicenter clinical trials are needed.
Summary
Besides hemodilution, the most common cause of anemia in pregnancy is iron deficiency. The gold standard for the diagnosis of iron deficiency anemia is a low ferritin level. The first-line treatment or iron deficiency anemia is oral iron. New evidence suggests that intermittent dosing is as effective as daily or twice-daily dosing with fewer side effects. For patients who cannot tolerate, absorb, or do not respond to oral iron, intravenous iron is preferred in the third trimester and sometimes as early as the second trimester. Iron supplementation decreases the prevalence of maternal anemia at delivery.
Iron deficiency anemia during pregnancy has been associated with an increased risk of low birth weight, preterm delivery, and perinatal mortality. Severe anemia with maternal hemoglobin levels less than 6 g/dL has been associated with abnormal fetal oxygenation resulting in non-reassuring fetal heart rate patterns, reduced amniotic fluid volume, fetal cerebral vasodilatation, and fetal death. Thus, maternal transfusion should be considered for fetal indications. All pregnant women should be screened for anemia and those with iron deficiency anemia should be treated with supplemental iron, in addition to prenatal vitamins. Patients with anemia other than iron deficiency anemia should be further evaluated by hematologist. Failure to respond to iron therapy should prompt further investigation and may suggest an incorrect diagnosis, co-existing disease, malabsorption (sometimes caused by the use of enteric-coated tablets or concomitant use of antacids), non-compliance, or blood loss.
References
- Auerback M, Abernathy J, Juul S, Short V, Derman R. Prevalence of iron deficiency in first trimester, non-anemic pregnant women. J Maternal-Fetal Neonatal Med 2021;34:1002-1005
- World Health Organization. The global prevalence of anemia in 2011. Available @ https://apps.who.int/iris/handle/10665/177094 Last accessed 20 June 2023
- World Health Organization. Haemoglobin concentrations, for the diagnosis of anaemia and assessment of severity. World Health Organization; 2011.
- Cook JD, Flowers CH, Skikne BS. The quantitative measurement of body iron. Blood 2003;101:3359-3364
- Cook JD. Diagnosis and management of iron-deficiency anemia. Best Pract Res Clin Haematol 2005;18:319-332
- Andrews NC. Pathology of iron metabolism. In: Hoffman R, Benz EJ Jr, Shattil S, et al, eds. Hematology: Basic Principles and Practice. 4th ed. New York, NY: Churchill Livingstone; 2004: 473-480
- Dugdale M. Anemia. Obstet Gynecol Clin North Am 2001;28:363-381
- Badfar G, Shohani M, Soleymani S, Azami M. Maternal anemia during pregnancy and small for gestational age: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2019;32:1728-1734
- Anemia in pregnancy. ACOG Practice Bulletin No. 233. American College of Obstetricians and Gynecologists. Obstet Gynecol 2021;138:e55-64
- Lee AI, Okam MM. Anemia in pregnancy. Hematol Oncol Clin North Am 2011;25:241-vii, vii.
- Jung J, Rahman MM, Rahman MS, Swe KT, et al. Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: a systematic review and metanalysis. Ann N Y Acad Sci 2019;1450:69-82
- Ko CW, Siddique SM, Patel A, Harris A, et al. AGA clinical practice guidelines on the gastrointestinal evaluation of iron deficiency anemia. Gastroenterology 2020;159:1085-1095
- Daru J, Allotey J, Pena-Rosas JP, Khan KS. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: a systematic review. Transfus Med 2017;27:167-174
- Jessani S, Saleem S, Hoffman MK, Goudar SS, et al. Association of haemoglobin levels in the first trimester and at 26-30 weeks with fetal and neonatal outcomes: a secondary analysis of the Global Network for Women’s and Children’s health’s ASPIRIN Trial. BJOG 2021;128:1487-1496
- Wiegersma AM, Dalman C, Lee BK, Karlsson H, Gardner RM. Association of perinatal maternal anemia with neurodevelopment disorders. JAMA Psychiatry 2019;76:1294-1304
- Rahmati S, Azami M, Badfar G, Parizad N, Sayehmiri K. The relationship between maternal anemia during pregnancy with preterm birth: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2020;33:2679-2689
- Zhou SJ, Gibson RA, Crowther CA, Baghurst P, Makrides M. Effects of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: long-term follow-up of a randomized controlled trial. Am J Clin Nutr 2006;83:1112-1117
- Cantor AG, Bougatsos C, Dana T, Blazina I, McDonagh M. Routine iron supplementation and screening for iron-deficiency anemia in pregnancy: a systematic review for the U.S. Prevention Services Task Force. Ann Inter Med 2015;162:566-576
- American College of Obstetricians and Gynecologists. Nutrition during pregnancy. Available @ and Last accessed on 3 July 2023 https://www.acog.org/store/
- Siu AL, U.S. Preventive Services Task Force. Screening for iron deficiency anemia and iron supplementation in pregnant women to improve maternal health and birth outcomes. U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015;163:529-536
- Achebe MM, Gafter-Gvili A. How I treat anemia in pregnancy: iron, cobalamin, and folate. Blood 2017;129:940-949
- Li N, Zhao G, Wu W, Zhang M., et al. The efficacy and safety of vitamin C for iron supplementation in adult patients with iron deficiency anemia: a randomized clinical trial. JAMA Netw Open 2020;3:e2023644.doi:1001/jama-networkopen.2020.23644
- National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheet. Available @ https://ods.od.nih.gov/factsheets/iron-HealthProfessional/ Last accessed 20 June 2023
- Dugdale M. Anemia. Obstet Gynecol Clin North Am 2001;28(2):363-381
- Bhavi SB, Jaju PB. Intravenous iron sucrose v/s oral ferrous fumarate for treatment of anemia in pregnancy: A randomized controlled trial. BMC Pregnancy and Childbirth 2017;17:137-147
- Shi Q, Leng W, Wazir R, Li J, et al., Intravenous iron sucrose versus oral iron in the treatment of pregnancy with iron deficiency anemia: a systematic review. Gynecol Obstet Invest 2015;80:170-178
- Auerbach M, Macdougall I. The available intravenous iron formulations: history, efficacy, and toxicology. Hemodial Int 2017;21(Suppl 1):S83-92
- Wang C, Graham DJ, Kane RC, Xie D, et al. Comparative risk of anaphylactic reaction associated with intravenous iron products. JAMA 2015;314:2062-2068
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol 2021;138:663-674
- Guinn NR, Cooter MI, Malsonave Y, Grimsley Y, et al. How do I develop a process to effectively treat parturients with iron deficiency anemia? Transfusion 2020;60:2476-2481
- Sifakis S, Pharmakides G. Anemia in pregnancy. Ann N Y Acad Sci 2000;900:125-136
- Carles G, Tobal N, Raynal P, Hersult S, Beucher G, et al, Doppler assessment of the fetal cerebral hemodynamic response to moderate or severe maternal anemia. Am J Obstet Gynecol 2003;188:794-799
- Sienas L, Wong T, Collins R, Smith J. Contemporary uses of erythropoietin in pregnancy: a literature review. Obstet Gynecol Surv 2013;68:594-602
- Sanchez-Gonzalez LR, Castro-Melendez SE, Angeles-Torres AC, et al. Efficacy and safety of adjuvant recombinant human erythropoietin and ferrous sulfate as treatment for iron deficiency anemia during the third trimester of pregnancy. Eur J Obstet Gynecol Reprod Biol 2016;205:32-36
- Saad AF, Stepanek R, Kothmann M, Wilson-Jimenez M, et al. Intravenous iron compared with oral iron supplementation for the treatment of postpartum anemia: a randomized controlled trial. Obstet Gynecol 2023;141:1052-1055
Publicado: 8 July 2023
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


