Consecuencias de la Marihuana y el Embarazo
WHEC Boletín Práctica Clínica y de gestión para proveedores de atención médica. Educación subvención prevista de Salud de la Mujer y el Centro de Educación (WHEC).
Marijuana is the most commonly used drug during pregnancy in the United States. The evolution of Cannabis sativa has been traced to the Central Asian / Himalayan region roughly 36 million years ago. Over time, cannabis spread to all regions with human habitation, reflecting the value placed on the medicinal, spiritual, and dietary utility of cannabis or marijuana. It was introduced to the United States as a medicinal product in the mid-1800s. And was widely prescribed by physicians as a therapeutic medicine until 1937, when sanctions were levied against medical or recreational use and physician prescribing marijuana. Prohibition culminated in 1970 with passage of the Controlled Substance Act, which formalized the criminalization of marijuana possession or use, regardless of quantity or context. Despite its illegal status, public demand for medical access led to the legalization of marijuana for medical use in California in 1996 and as of 2014, voters in an additional 24 states and the District of Columbia have followed suit. Marijuana is the most frequently used illicit drug in Western countries. Popular demand and legal access to medical marijuana began despite the lack of well-designed randomized clinical trials (RCTs), and it is the result of decades-long federal law enforcement obstruction. However, numerous RCTs have been published since 2000, markedly clarifying appropriate indications and contraindications. The sensationalized 1980s theory of marijuana use as the gateway to hard drug use lacks empirical support. While heavy adolescent use is associated with risk of other drug abuse, there is no good evidence of causality or directionality. Additional research is necessary to clarify this point.
The purpose of this course is to provide healthcare professionals with unbiased and evidence-based information regarding the use of marijuana and other cannabinoids for the treatment of medical conditions. The review discusses the history of therapeutic cannabis use; outlines the function of the endocannabinoid system; analyzes the pharmacology of exogenous cannabinoids in clinical or experimental use; discusses potential side effects and areas of safety concern when medicinal cannabis and other cannabinoids are used. It also describes the well-confirmed and less-confirmed indications of therapeutic cannabinoid use; and identifies primary indications, side effects, chronic effects, and contraindications to therapeutic cannabinoid use. A large body of clinical trials has now been published on cannabis and other cannabinoids in the treatment or management of a wide range of diseases and conditions. The review suggests a need for healthcare provider training on potential consequences of perinatal marijuana use and communication skills for counseling patients about perinatal marijuana. An increasing number of states are passing or considering medical marijuana laws. The goal of this document is to address the public health system's responsibility to educate physicians and public about the impact of marijuana on pregnancy and to establish guidelines that discourage the use of medical marijuana by pregnant women or women considering pregnancy.
TERMS
The following terms are used often in discussions of medical marijuana use, and these definitions may help clarify the issues being described.
Cannabis (THC): derived from Cannabis sativa, the proper name of the marijuana plant. Cannabis is a dioecious species, meaning it has male and female plants. Roughly half the plants grown from seed are female; when not fertilized by males to produce seeds, female plants bear flowering buds called sinsemilla, the part of the plant with highest Delta9-tetrahydrocannabinol (THC) or delta9- tetrahydrocannabinol (THC) concentration (1). THC is the primary psychoactive ingredient, depending on the particular plant.
Marijuana: a synonym and slang term for cannabis, often used when discussing medical use. It is also called: ganja, grass, hash, pot and weed.
Cannabinoid: a category that includes endogenous cannabinoid receptors, their endogenous ligands, and the plant-occurring or synthetic molecules that interact with cannabinoid receptors or their ligands (2).
Cannabidiol (CBD): THC and CBD are the most abundant cannabinoids, depending on the particular plant. The relative concentration of THC, CBD, and other cannabinoids in a given plant is influenced by cannabis strain, soil and climate conditions, and cultivation techniques. Outside of the United States, CBD is available in equal ratio to THC in the oromucosal spray nabiximols. In Canada and the Netherlands, some cannabis strains available for medicinal use have been bred to overexpress CBD, for a 1:1 ratio of CBD to THC.
Δ9-tetrahydrocannabinol: the primary active cannabis constituent. It is referred throughout in this review as THC.
PREVALENCE
In 2013, 19.8 million, or 7.5% of the US population, reported its use, an increase from 2007 when only 5.8% of the population has used marijuana within the past month (3). Reported prevalence rates of marijuana use in pregnancy vary from as low as 3% to as high as 34% (4). In the 2009 National Survey on Drug Use and Health report, 4.6% of women reported they used marijuana during pregnancy, whereas population-based studies using biochemical testing noted rates as high as 12% (4). We anticipate an increase in marijuana use in pregnancy as legalization of marijuana increases throughout the United States. Although no epidemiological studies of the use of marijuana during pregnancy provide information as to the source of the women's access to marijuana, a recent report from the U.S. Drug Testing Laboratories (Chicago, IL), examined Colorado's 2012 ballot initiative allowing large-scale marijuana production and statewide distribution and studied its impact on patterns of medical marijuana use. The ballot initiative was passed in November 2012 and went into effect January 2014.
Based on local hospital protocols, meconium specimens from newborns across the nation that were determined to be at high risk of prenatal drug or alcohol exposure were collected and forwarded to the US Drug Testing Laboratories for analysis. Data were analyzed for the presence of marijuana in specimens originating from hospitals within the state of Colorado vs specimens sent from the rest of the United States during the first 9 months of years 2012 and 2014. Positive samples were confirmed for 9-carboxy- Delta9-tetrahydrocannabinol using gas chromatography-mass spectrometry. The rates of positive meconium samples for marijuana were similar at each of the time points in the 2 populations, with an approximately 10% increase in the rate of positive marijuana samples in Colorado and in the rest of the country. More importantly, however, although the concentration of marijuana in exposed neonates' meconium for the US-wide population demonstrated little change across the two-time periods, the exposed neonates in Colorado experienced substantially more exposure to marijuana in the post-legalization period as indicated by a significant increase in the concentrations (7).
Cannabis Addiction: Roughly 9%, or 1 out of 11, who use recreational marijuana will develop an addiction syndrome; the figure increases to 17%, or 1 out of 6, who begin use in their early teens (8). This compares with lifetime prevalence rates of 32% for nicotine, 23% for heroin, 17% for cocaine, and 15% for alcohol (8). Addiction risk among medical cannabis users is unknown. Data on cannabis addiction and risk factors come primarily from recreational users who began during adolescence or early adulthood and used high-potency cannabis with great frequency and intensity in the absence of medical supervision. Whether these data apply to the typically older adult patient using smaller doses of medical marijuana for symptom control is not known. The psychoactive effects and potential abuse liability of recreationally used cannabis are well known, but little is known of this potential with nabiximols spray (equal-ratio THC and CBD). A safety analysis using all published and unpublished nabiximols RCTs found that intoxication scores were low (9). Euphoria was reported by only 2.2% of subjects, development of tolerance was not documented, abrupt cessation did not result in a withdrawal syndrome, and no cases of abuse or diversion were reported. An abuse liability study of nabiximols in experienced recreational cannabis smokers found some abuse potential at higher doses relative to placebo, but consistently lower abuse liability than equivalent doses of pure THC (9). Although medical marijuana laws in some states have been anecdotally linked to increased recreational use among adolescents, a 2013 evaluation of the effects of these laws on adolescent marijuana use from 2003 through 2011 found that they had no measurable effect (9).
LEGALIZATION OF MARIJUANA
Currently both recreational and medical marijuana remain illegal by federal law in the United States. However, the legalization of medical and recreational marijuana at the state level is increasing throughout the United States. At this point in the United States, 28 states have legalized the use of medical marijuana, 16 states allow limited medical use of marijuana. 8 states (Alaska, California, Colorado, Massachusetts, Nevada, Oregon, Washington and Washington, D.C.) have legalized both medical and recreational marijuana. In general, the legislation in all states removes state-level criminal penalties on the use, possession, and cultivation of marijuana by patients who possess written documentation from their physician advising that they would derive benefit from the medical use of marijuana. Only Oregon has legislation that requires a point-of-sale warning at dispensaries regarding cannabis use in pregnant or breast-feeding women. The Colorado Department of Health has posted recommended screening questions for women who are pregnant and recommends discussing the importance of the cessation of marijuana during pregnancy or, at a well-woman visit, if a woman desires to become pregnant. As states continue to legalize marijuana, making it more accessible, increased use across the general population could lead to increased rates of prenatal marijuana exposure, especially because most women do not realize they are pregnant during the first weeks after conception.

The Colorado Experience: Medical marijuana was legalized in Colorado in the year 2000. However, it was not until 2009 when the US Attorney General issued a statement passing the jurisdiction of marijuana law enforcement to state governments that we saw a sharp increase in the number of medical marijuana uses in the United States (5). In 2012, recreational marijuana was legalized in the state of Colorado with the passing of Amendment 64. There is no stipulation in the law stating that pregnant women cannot purchase or possess marijuana. Sales of recreational marijuana have been steadily increasing since the opening of the first recreational dispensaries on 1 January 2014. The state of Colorado does not publish overall sales amounts but does publish tax revenue on a monthly basis. In January 2014, the revenue was 3.5 million dollars. The monthly tax revenue is now up to 7.6 million dollars for the month of October 2014, showing a steady increase in sales and consumption (6). In addition, there has been an increase in the use of alternative forms of consumption such as vaporizing (heating the cannabis to release THC and cannabinoids without making it smoke), lotions, and edibles (5),(7).
Following the legalization of marijuana, we have noted several unanticipated adverse consequences of the increase in marijuana availability including an increase in pediatric overdoses and emergency visits for marijuana toxicity (5). When women have been followed up longitudinally during pregnancy, a decrease in marijuana use has been noted across trimesters of pregnancy. In a 1 year prospective cohort study, marijuana use in pregnancy declined from 32% in the first trimester to 16% in the third trimester (10). Similarly, a longitudinal prospective study on drug use in pregnancy (n=86), the Development and Infancy Study, found that the percentage of women who used marijuana throughout the pregnancy declined. However, approximately 60% of women who used marijuana in the year prior to pregnancy continued to use more than 10 joints per week, indicating that many women continue use throughout pregnancy (11). It should be noted that the women in this study smoked an average of 21 joints per week in the month prior to pregnancy and may not be representative of less frequent users of marijuana.
The United Kingdom (UK) Experience: Two thirds of adults surveyed in a UK study noted that cannabis was either "not very harmful" or "not at all harmful" (12). This is in contrast to other recreational drugs such as heroin or cocaine in which less than 5% of adults surveyed perceived them to be either "not very harmful" or "not at all harmful". The perceived safety likely contributes to the high prevalence of its use in pregnancy.
THE ENDOGENOUS CANNABINOID SYSTEM
The endogenous cannabinoid system (ECS) is a signaling system that includes cannabinoid receptors, endogenous receptor ligands (termed endocannabinoids), and their synthesizing and degrading enzymes (13). Core functions of the ECS have been described as "relax, eat, sleep, forget, and protect," shorthand for the diversity of processes involving the ECS. The ECS regulates neuronal excitability and inflammation in pain circuits and cascades and also helps regulate movement, appetite, aversive memory extinction, hypothalamic-pituitary-adrenal (HPA) axis modulation, immunomodulation, mood, wake / sleep cycles, blood pressure, bone density, tumor surveillance, neuro-protection, and reproduction. The so-called "runner's high" and the effects of osteopathic manipulative therapy and electro-acupuncture are mediated by the ECS. The ECS is a system common to all vertebrates and many invertebrates and has been present in living organisms as far back as 600 million years. In the invertebrate species Hydra vulgaris, a primitive evolutionary throw-back to several hundred million years, feeding is mediated by the ECS. This discovery underscores the essential pro-survival function of the ECS that long pre-dates mammalian evolution, where the more recently evolved hypothalamic system regulates the survival function of appetite (14).
Cannabinoid Receptors:
CB1 Receptors: CB1 receptors are the most abundant G-protein-coupled receptors in the brain and are expressed at lower densities in many peripheral tissues. CB1 receptors solely mediate the psychotropic and behavioral effects of cannabinoids and regulate several peripheral processes, such as energy homeostasis, cardiovascular function, and reproduction. CB1 distribution in the brain matches the known pharmaco-dynamic effects of cannabinoids; CB1 activation prominently modulates cognition and memory, perception, control of motor function, and analgesia (15).
CB2 Receptors: CB2 receptors are sparsely expressed in the central nervous system (CNS) but highly expressed in immune cells, where they play an important role in regulating immune function and inflammation. Their activation modulates immune cell migration and cytokine release, and CB2 receptor expression on CNS microglia may explain cannabinoid efficacy in reducing cytokine-mediated neuro-inflammation (16).
Other Endocannabinoid Receptors: In addition to CB1 and CB2 receptors, endocannabinoids are thought to bind several other molecular targets. These include a third presumed cannabinoid receptor, GPR55 (sometimes termed CB3), the transient receptor potential cation channel subfamily V (TRPV1), and a class of nuclear receptors / transcription factors known as the peroxisome proliferator-activated receptors (PPARs) (13).
Endogenous Cannabinoids Receptor Ligands: Anandamide and 2-arachidonoyl glycerol (2-AG) are the two primary endogenous cannabinoids receptor ligands.
PHARMACEUTICAL CANNABINOID PREPARATIONS
Following identification of THC as the primary active constituent in cannabis, investigative focus primarily involved the therapeutic potential of isolated THC. Although efficacy was found across many pathologic conditions, the prominent psychotropic effects of THC limited its clinical appeal. Discovery of the ECS and characterization of additional phytocannabinoids prompted research evaluation of the therapeutic potential of other phytocannabinoids lacking the psychotropic effects of THC. Investigation of CBD, cannabigerol, Delta9-tetrahydrocannabivarin, and cannabidivarin led to promising results in preclinical models of CNS disease. This research also revealed the basis for expanded receptor targeting beyond central brain (CB) receptors with these agents and the suggestion of clinical utility in epilepsy, neurodegenerative diseases, affective disorders, and central modulation of feeding and appetitive behavior (17). These findings have influenced the direction of modern cannabinoid drug development and evaluation. Many novel cannabinoid therapeutics are in early-stage safety and efficacy evaluation, and the following cannabinoids are in current clinical or advanced-phase investigative use: Dronabinol (branded as Marinol), Nabilone (Cesamet), Nabiximols (Sativex), Cannador, and Pharmaceutical-Grade Smoked Cannabis.
Distribution: THC distribution is time-dependent and begins rapidly after absorption. In plasma, THC is 95% to 99% plasma protein bound, primarily lipoproteins. The tissue distribution of lipophilic THC and its metabolites mostly involves uptake in fatty tissues and highly perfused organs such as the brain, heart, lung, and liver. Whether THC accumulates in the brain with long-term use is unknown, due to limits in THC access and accumulation imposed by the blood-brain barrier (18).
Adverse Drug-to-Drug Interactions: Most patients in the RCTs discussed in this course were maintained on their pre-study medications for neuropathic pain, cancer pain, fibromyalgia, or multiple sclerosis. In these and other RCTs, patients smoked or ingested cannabis while taking their prescribed opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), muscle relaxants, ketamine, anticonvulsants, antidepressants, and benzodiazepines. Cannabis use with these other agents was well tolerated, and observed side effects did not differ from those expected with cannabis (19). In theory, ingesting cannabis with drugs that alter its metabolic pathway should increase the risk of side effect enhancement or efficacy failure, but adverse drug-to-drug interactions of clinical relevance have not been reported to date. Cannabis should be used with caution by patients also using sedating substances such as alcohol or benzodiazepines (19).
Tolerance: Tolerance is defined as tissue adaptation resulting from repeated drug exposure, such that one or more drug effects diminish over time. Cannabis tolerance primarily results from pharmacodynamic mechanisms, including changes in CB1 signaling ability due to receptor desensitization and down-regulation. THC tolerance varies across different brain regions, possibly explaining why tolerance develops to some cannabis effects but not to others. Tolerance to most THC effects develops after a few doses and then disappears rapidly following cessation, and pharmacodynamic tolerance can be minimized by combining a low dose of cannabinoid with one or more additional therapeutic drugs (20).
SIDE EFFECTS AND SAFETY
Information on medical cannabis safety and side effects should ideally come from RCTs that control for confounding factors that may otherwise account for the results. Such studies are increasingly being published, but similar to other drug efficacy trials, safety information is available with short-term (less than 3 months) use while long-term safety data remains sparse. In contrast to studies with medicinal users, many studies of long-term heavy recreational users have been published. Generalizing safety outcomes from chronic recreational users to medicinal users is cautioned against because of numerous confounding factors, including differences in age of first regular use; duration, quantity, and THC content of cannabis use; concurrent alcohol or other drug use; drug delivery approaches; and past or current psychiatric, neurologic, and comorbid medical histories (21). Raphael Mechoulam, who in 1964 co-discovered THC, concluded that most cannabis safety data from "street users" is "useless" (his words) for extrapolation to medicinal cannabis safety, based on the before-mentioned factors and the widely variable THC and unknown CBD content of illicitly obtained cannabis in contrast to cannabis now cultivated under tightly controlled environmental conditions to ensure reliability (21). In the following sections, the available evidence on medical cannabis and pharmaceutical cannabinoids is presented.
RISK / BENEFIT CONSIDERATIONS
Importantly, the potential acute and long-term adverse effects with medical cannabis should be weighed against the known side effect profiles of standard therapeutic agents for the same indication (22). For example, in standard therapies for chronic pain or spasticity, opioids often produce sedation, nausea, constipation, physiological dependence, and a substantially more severe withdrawal syndrome than cannabis withdrawal. Tricyclic antidepressants and antiepileptic drugs are frequently prescribed for chronic neuropathic pain and may produce sedation, constipation, dizziness, palpitations, visual disturbance, urinary retention, and neuromuscular effects. Antispasmodic drugs may produce sedation (e.g., baclofen), hypotension (e.g., tizanidine), and potentially serious interactions with antibiotics (as with tizanidine and ciprofloxacin). Benzodiazepines prescribed for spasticity may produce sedation, psychomotor incoordination, memory impairment, paradoxical reactions, dependence, and with daily long-term use, a severe protracted withdrawal syndrome. Opioids and benzodiazepines are also drugs with potential for abuse, addiction, diversion, and fatal overdose exceeding cannabis. This comparison helps put consideration of the relative benefits and risks of medical cannabis in the proper context (22). As with any drug therapy, important considerations include the dose-response relationship and margin of safety that separates beneficial dose from dosage producing adverse effects. Safety concerns can be addressed, as with any drug, by appropriate patient screening and monitoring, adherence to known contraindications, and administration with alternative delivery systems (as in patients with lung disease). In many (non-cannabis) contexts, clinical medicine involves balancing risk and benefit even when limited evidence is available to base a decision, and the needs and wishes of patients should be considered while the merits of medical cannabis use are debated.
PHARMACOLGIC MANAGEMENT OF CANNABIS SIDE EFFECTS
Results from RCTs of smoked cannabis found that side effects were generally dose-related, mild-to-moderate in severity, time-limited, and less common in experienced cannabis users. Most frequent were dizziness or lightheadedness (30% to 60% of subjects), dry mouth (10% to 25%), fatigue (5% to 40%), muscle weakness (10% to 25%), myalgia (25%), and palpitations (20%). Cough and throat irritation occurred initially in a few participants. Euphoria was reported in some but not all subjects, with the low incidence attributed to plasma THC concentrations less than 25% of the levels generally found with recreational cannabis use. Infrequently, tachycardia and postural hypotension were noted, a potential concern in patients with cardiovascular disease. Tachycardia was a frequent acute physiological effect, with it and other acute cardiovascular effects rapidly resolving due to the brief period of THC occupancy and then distribution out of the circulatory system (23).
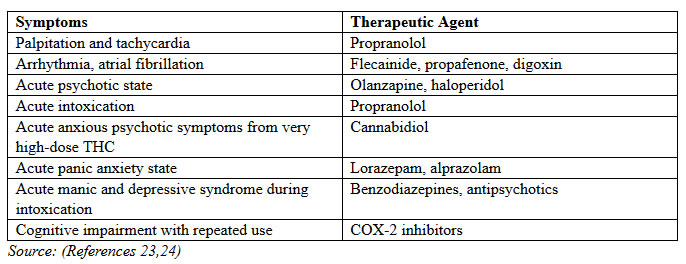
AREAS OF SAFETY CONCERNS
Contaminants in the Cannabis Plant
Cannabis may be contaminated by a variety of organisms, such as Aspergillus fungus and bacteria that can result in fulminant pneumonia, especially in immunocompromised persons. Non-biologic contaminants can include heavy metals such as aluminum and cadmium from the soil, with cadmium readily absorbed into the plant at high concentrations. Organophosphate pesticides are found less often in cannabis grown outdoors versus indoor cultivation (25). Concerns over inorganic and biologic contaminant ingestion prompted Health Canada and the Office of Medicinal Cannabis (OMC) to carefully control all aspects of cultivation, test the product for the presence of mold spores and 28 different metals including heavy metals, and pre-emptively irradiate all cannabis products before distribution to medical or research users. This is not currently done to most cannabis available in the United States.
Pulmonary Function
Physician and patient concerns over pulmonary harm from cannabis smoking have been based on the known hazards from smoking tobacco, findings of carcinogenic compounds in cannabis smoke, and earlier epidemiological studies associating long-term cannabis use with respiratory dysfunction (26). This has contributed to reluctance over medical smoked cannabis use, but more recent scientific data challenge these assumptions. Although many carcinogens and tumor promoters are common to tobacco and cannabis smoke, differences in the active constituents result in different biological outcomes. Molecules in tobacco smoke enhance carcinogenic pathways through several mechanisms, including circumvention of normal cellular check-point protective mechanisms; activation of respiratory epithelial cell nicotine receptors; promotion of tumor angiogenesis; stimulation of enzymes that convert polycyclic aromatic hydrocarbons found in smoke into carcinogens; and prevention of apoptotic cascades (cell death) in cells accumulating sufficient genetic damage. In contrast, molecules in cannabis smoke inhibit carcinogenic pathways through down-regulation of immunologically-generated free radical production (the innate response to inhaled smoke and particulate); THC blockade of enzymatic conversion of smoke constituents into carcinogens; the absence of cannabinoid receptors in respiratory epithelial cells (which maintains DNA damage checkpoint mechanism integrity with prolonged cannabis smoke exposure); and the anti-angiogenic, tumor-retardant, and anti-inflammatory activity of many cannabinoid smoke constituents (26).
These factors appear in the results of a 20-year longitudinal study of pulmonary health in 5,115 participants who smoked cannabis. The authors stated that pulmonary risks from cannabis smoking had been overstated and found that, unlike tobacco smoking, cannabis smoking had no effect on measures of pulmonary function. Medicinal use of smoked cannabis was also found to be very unlikely to produce adverse effects on pulmonary function. In 878 Canadians 40 years of age and older, history of tobacco smoking or tobacco and marijuana smoking, but not marijuana-only smoking, significantly elevated the risk of respiratory problems or chronic obstructive pulmonary disease (COPD) relative to non-smokers (27). Vaporizing systems have been developed to further minimize pulmonary risks from smoked cannabis. These involve heating the plant material short of combustion and then inhaling the mist (instead of smoke). Vaporization may produce smaller quantities of the toxic smoking byproducts carbon monoxide, polycyclic aromatic hydrocarbons and tar, and compared with smoked cannabis, vaporization was found to significantly reduce carbon monoxide levels.
Immunosuppression
Concern was raised in the 1990s over the potential negative effects of cannabinoids on immune function in immunosuppressed patients, particularly those with HIV. Data from several studies have alleviated these concerns. In HIV patients randomized to placebo, dronabinol, or smoked cannabis for 21 days, both cannabinoid groups failed to show increased viral load or reductions in protease inhibitor levels or CD4 or CD8 cell counts. Both cannabinoid groups showed statistically significant weight increases, and the smoked cannabis group showed significantly increased CD4 and CD8 counts (28). Supportive data include a study of primates injected daily with THC before and after infection with simian immunodeficiency virus (SIV). Contrary to expectations, chronic cannabinoid exposure did not increase viral load or diminish immune function. Instead, the primates given THC showed significantly decreased rates of early mortality from SIV infection, associated with attenuation of plasma and cerebrospinal fluid viral load and retention of body mass. Other conformational findings include a 10-year follow study of HIV patients, which found that regular cannabis smoking had no effect on viral load or CD4 and CD8 cell percentages (28). An exception comes from preclinical trial results suggesting that increased CB2 activity may impose risks in immunocompromised patients with specific infection, such as Legionella.
Neurocognitive Impairment
Abundant evidence from adult subjects shows an impairing acute effect of smoked cannabis on verbal and working memory for several hours after ingestion, mitigated by THC dose, ratio of THC: CBD and genetic vulnerability factors (29). Research increasingly suggests that while cognitive impairment from long-term cannabis use in adults is mostly reversible following cessation and weekly quantity potentially contributes to cognitive deficit, long-term early-onset cannabis use is associated with greatest morphological and functional alterations in the still-developing brain. This effect has not been found in all studies, and a possible confounding factor is differences in the CBD to THC ratio, as smoked cannabis with high CBD content protects against the memory-impairing effects associated with high-THC cannabis strains (29). While cognitive function in long-term medical cannabis users has not been evaluated, a review of the published research on short- and long-term cognitive function in recreational users suggests that cognitive impairment is unlikely to persist beyond the acute intoxication state, even with high-THC cannabis, in late-onset users, short-term users, and occasional users.
Amotivational Syndrome
Amotivational syndrome is not a medical diagnosis but a term used to describe adolescents and young adults who lose interest in and drop out from school, work, socializing, and other goal-directed activities. Cannabis has been cited as the cause when its heavy use accompanies these symptoms, but evidence of causality is lacking (29).
Schizophrenia and Psychoses
An acute psychotic reaction to cannabis has been described and is more likely to occur in young adults who are under stress and have a pre-existing vulnerability to psychoses or schizophrenia. An association has been found between cannabis use history and schizophrenia, but the causal direction of this link has not been established, with many studies suggesting causality showing instead a non-specific association between the most severe levels of cannabis use and a wide range of adverse psychosocial outcomes (30). Furthermore, cannabis use in the general population soared between 1949 and 1995, while the population rates of schizophrenia remained stable (30). However, a subgroup of patients who are genetically vulnerable to cannabis-induced acute psychoses, and possibly cannabis-initiated schizophrenia, carry a functional polymorphism in the catechol-O-methyltransferase gene and a polymorphism in the brain-derived neurotrophic factor gene. Considering the potentially substantial risks, cannabis should be avoided in adolescents and adults with current, past, or family history of any psychotic disorder.
Toxicity and Overdose
There are no cases in the literature of death due to toxicity following the maximum oral THC dose in dogs (up to 3,000 mg/kg THC) and monkeys (up to 9,000 mg/kg THC). In animals and humans, it is virtually impossible to induce fatal toxicity, and no human fatalities resulting from cannabis ingestion have been documented to date. The side effect profile of medical cannabis is comparable to those produced by other medications tolerated by patients and approved for clinical use by the U.S. Food and Drug Administration (FDA). The rare acute complications resulting in emergency department presentation, such as panic attacks, psychosis, or convulsions, can be managed with conservative measures such as reassurance in a quiet environment and intravenous administration of benzodiazepines if needed. The greatest risk for toxicity and potential overdose is among children who may consume cannabis edibles, beverages, or candies inadvertently (31). In adults, most toxic reactions are mild, but in children, overdose can result in significant respiratory depression (31). Signs can include somnolence, hallucinations, dyspnea, CNS depression, and even coma. Healthcare professionals should assess for availability of cannabis in the household if these signs present with no known explanation. If necessary, airway management and ventilation may be administered.
Cannabis Withdrawal Syndrome
Until recently, considerable doubt surrounded the possibility of a cannabis withdrawal syndrome, however, cannabis withdrawal syndrome has now been unequivocally demonstrated in heavy chronic recreational users (32). With abrupt cessation, withdrawal symptoms emerge within 1 to 2 days, reach peak intensity after 2 to 6 days, and generally resolve within 1 to 2 weeks. Common symptoms include irritability or anger, nervousness, tension, restlessness, reduced appetite, insomnia and sleep difficulties, dysphoria, and craving. Less frequent symptoms are chills, stomach pain, shakiness, and sweating (32). Cannabis withdrawal can resemble a low-grade opioid withdrawal but usually lacks the severe aches and pains, piloerection, diarrhea, sweating, stuffy nose, and muscle spasms common to opioid withdrawal. The severity of cannabis withdrawal, and whether it develops at all in strictly medical users, is unknown. With cessation of regular medical use, the pharmacokinetics and possibly pharmacodynamics of THC, such as slow elimination, may diminish withdrawal symptom manifestation into the subclinical level of severity.
Cannabis Addiction
Roughly 9%, or 1 out of 11, who use recreational marijuana will develop an addiction syndrome; the figure increases to 17%, or 1 out of 6, who begin use in their early teens. This compares with lifetime prevalence rates of 32% for nicotine, 23% for heroin, 17% for cocaine, and 15% for alcohol (33). Addiction risk among medical cannabis users is unknown. Data on cannabis addiction and risk factors come primarily from recreational users who began during adolescence or early adulthood and used high-potency cannabis with great frequency and intensity in the absence of medical supervision. Whether these data apply to the typically older adult patient using smaller doses of medical marijuana for symptom control is not known. The psychoactive effects and potential abuse liability of recreationally used cannabis are well known, but little is known of this potential with nabiximols spray (equal-ratio THC and CBD). A safety analysis using all published and unpublished nabiximols RCTs found that intoxication scores were low. Euphoria was reported by only 2.2% of subjects, development of tolerance was not documented, abrupt cessation did not result in a withdrawal syndrome, and no cases of abuse or diversion were reported. An abuse liability study of nabiximols in experienced recreational cannabis smokers found some abuse potential at higher doses relative to placebo, but consistently lower abuse liability than equivalent doses of pure THC (33). Although medical marijuana laws in some states have been anecdotally linked to increased recreational use among adolescents, a 2013 evaluation of the effects of these laws on adolescent marijuana use from 2003 through 2011 found that they had no measurable effect (33).
INDICATIONS AND PRACTITIONER CONSIDERATIONS
Since 2000, there has been a significant increase in the quantity and quality of cannabis efficacy studies. Cannabis and other cannabinoids are seldom considered first-choice therapeutic options but are used instead in patients for whom standard therapies are ineffective or intolerable either as sole therapy or more typically as an add-on to the current regimen. Safety profile of cannabis versus opioids suggests that it can be a valuable addition to therapeutic options for: chronic pain; neuropathic pain; nociceptive pain; reducing opioid requirements; neuropsychiatric disorders - multiple sclerosis and spasticity, post-traumatic stress disorder (PTSD), seizure disorders, fibromyalgia; gastrointestinal disorders / dysfunction - irritable bowel syndrome, nausea and vomiting, hepatitis C therapy; sleep disorders; cancer and HIV-associated anorexia and weight loss and glaucoma.
As noted, cannabis is generally recommended for patients in whom standard therapies have been ineffective or intolerable. Appropriate indications for medical cannabis have most recently been formalized by the State of New York, the OMC in the Netherlands, and Health Canada and include (34):
- Disorders of pain and spasticity, including intractable spasticity, multiple sclerosis, and spinal cord damage or injury;
- Chronic neuropathic pain, including nerve damage, phantom limb pain, facial neuralgia, and post-therapeutic neuralgia;
- Pain from cancer and HIV/AIDS;
- Nausea and vomiting from chemotherapy, radiotherapy, and/or medication for HIV and hepatitis C;
- Neuropsychiatric disorders, including tics associated with Tourette syndrome, epilepsy, neuropathy, Parkinson's disease, and PTSD;
- Autoimmune conditions, including arthritis, lupus, and Crohn's disease;
- Palliative treatment of cancer and AIDS to stimulate appetite, avoid weight loss, and reduce debilitation and wasting syndrome;
- Treatment-resistant glaucoma;
- A debilitating symptom associated with a medical condition or the medical treatment of that condition, other than those described above.
Dosage and Administration Guidance
The ideal dosage of cannabis or THC varies by condition and patient characteristics. For the treatment of refractory pain, the recommended daily dose of inhaled or ingested cannabis is 2.5 g for refractory pain and not more than 5 g for other indications (e.g., nausea and vomiting, anorexia); larger doses are divided to two or three doses per day (35). Studies conducted in Israel and the Netherlands found the average dose for patients in their medical cannabis programs was 1.5 g/day and 0.68 g/day, respectively (35). The recommended initial dose of dronabinol is 2.5 mg at bedtime. This may be titrated up to effect to a maximum of 20 mg per day. Nabilone for chemotherapy-induced nausea and vomiting is started at 1-2 mg twice daily and may be increased to a maximum of 6 mg/day in three divided doses. In all cases, it is important to begin with the lower dose in the range and increase if needed. If the starting dose is tolerated but the desired effects are not achieved, slowly increase the dose. One should keep in mind that the therapeutic dose is usually lower than the recreational dose. For medicinal purposes, the OMC recommends vaporized or oral ingestion; smoking is not recommended. Patients orally ingesting cannabis or cannabinoids should be advised of the slow onset and the need to ingest small amounts spaced several hours apart.
Vaporizing: Though it is often recommended in discussions of medical marijuana use, many healthcare professionals are not familiar with the process of administering cannabis through vaporizing. In essence, active cannabis ingredients can be vaporized if cannabis is heated and inhaled without combustion. The right temperature is reached when vapor is just visible as a light mist, but no smoke has formed, usually at a temperature of 180 degC to 195 degC. Using this method, the same cannabis can be used two to three times. In most cases, the recommended initial dosing is one to two times per day, with a minimum of 5 to 15 minutes between inhalations. Patients may need to inhale a few times, until the desired effect is reached or side effects occur. It may take up to two weeks to achieve steady-state THC concentrations and full therapeutic effect.
Tea: As discussed, a cannabis tea may be used to ingest medical marijuana, though the limited THC bioavailability and lack of water solubility make this a less attractive option in most cases. To brew the cannabis tea, 0.5 g cannabis in boiled in a pint of water for 15 minutes. The plant material is then strained out of the tea and sweeteners are added. The addition of a substance containing fat (e.g., milk powder) can improve the availability of THC in the tea. The tea may be kept refrigerated for up to five days. The usual initial dose is 1 cup in the evening, though if the effects are insufficient after two weeks, an additional cup (usually in the morning) may be added.
CONTRAINDICATIONS of CANNABIS USES
At this time, experts recommend limiting medical cannabis use to adults older than 18 years of age (36). There are several other contraindications to the use of medical marijuana, including:
- Current, past or family history of schizophrenia or other psychotic disorders;
- History of hypersensitivity to cannabinoids or smoke;
- Severe cardiopulmonary disease;
- Severe liver or renal disease;
- Pregnancy or planned pregnancy;
- Breastfeeding.
Cannabis may be considered with caution for patients with the following factors when alternatives have been ineffective / poorly tolerated, the benefit / risk ratio closely evaluated, and with sufficient monitoring (36):
- Smoked cannabis in patients with asthma or COPD;
- History of substance abuse;
- Non-psychotic psychiatric condition (e.g. anxiety, panic attacks);
- Current CNS depressant therapy.
MATERNAL MARIJUANA USE AND ADVERSE NEONATAL OUTCOMES
It is generally assumed that marijuana is one of the more widely used controlled substances during pregnancy. However, there remains a general paucity of population-based data regarding its use and subsequent perinatal morbidity. Direct patient query during pregnancy regarding marijuana, tobacco, and nicotine use would provide crucial population-based data on perinatal risk. This study sought to examine maternal and neonatal outcomes in pregnancies with reported marijuana exposure, in isolation or in combination with maternal cigarette smoking (37). In this study, after controlling for potential confounders, while marijuana exposure alone was not associated with significant perinatal adverse outcomes, co-use with cigarette smoking rendered increased risk over either alone (37). Due to observed prevalence of concurrent cigarette and marijuana use, it is likely importance to counsel patients regarding use in pregnancy.
Prior studies of varying quality and methodology investigating neonatal outcomes related to marijuana use in pregnancy have yielded conflicting results, some showing increased risks for adverse neonatal outcomes whereas others show no increased risk. Many are hampered by selection bias by using volunteer cohorts and classification bias by defining marijuana exposure through self-report. Additionally, previous studies do not uniformly adjust for confounding as a result of coexistent tobacco use, a vital consideration when investigating health outcomes associated with marijuana use, because marijuana is often used in combination with tobacco (38). In conclusion, the results of this systematic review and meta-analysis suggest that the increased risk for adverse neonatal outcomes reported in women using marijuana in pregnancy is likely the result of coexisting use of tobacco and other cofounding factors and not attributable to marijuana use itself. Although these data do not imply that marijuana use during pregnancy should be encouraged or condoned, the lack of a significant association with adverse neonatal outcomes suggests that attention should be focused on aiding pregnant women with cessation of substances known to have adverse effects on the pregnancy such as tobacco.
In this study, obstetric health care provider responses to disclosure of marijuana use occurred in approximately half of patient encounters when marijuana use was disclosed and focused on legal and procedural consequences with less focus on health or medical implications (39). The results suggest a need for health care provider training on potential consequences of perinatal marijuana use and communication skills for counseling patients about perinatal marijuana. There is a high rate of absent and insufficient health care provider responses to pregnant women's disclosure of marijuana use during the first obstetric visit. Additionally, when counseling occurred, discussions focused on potential legal or child protective services implications rather than potential medical or pregnancy consequences. Health care providers may, like the general public, have relatively favorable attitudes toward marijuana use compared with other illicit drugs. The U.S. population has demonstrated increasingly favorable attitudes regarding marijuana use. From 2010 to 2013, Pew Research surveyed and 11% increase in the proportion of supporters for marijuana legalization with 52% favoring legalization. Furthermore, the survey noted 48% of Americans tried or used marijuana in their lifetime, up 38% from a decade (40). The survey of family physicians in Colorado found that 30% favored legalization for recreational use and 27% believed there were significant physical benefits to using marijuana (40).
PERINATAL COUNSELING
Counseling should address the potential medical risks for both mother and fetus and strategies to assist the patient in quitting. Furthermore, studies are needed to better understand the beliefs, perspectives, knowledge, and concerns of both pregnant patients and obstetric health care providers to develop and tailor effective communication resources and training interventions on perinatal marijuana that address the specific needs and concerns of health care providers and patients in the varying regions across the nation. To inform future policies regarding perinatal marijuana use, it is imperative to understand the effects of legalization on beliefs, attitudes, behaviors, practices, and concerns regarding marijuana use among pregnant patients and their obstetric health care providers.
Suggested Marijuana Counseling
Implications for mother and child:
- Small gestational weight, preterm delivery;
- Respiratory complications (asthma, lung disorders);
- Cognitive deficits (attention, memory, learning, and behavioral);
- Higher rates of anxiety and depression.
Support for patients:
- Available medications to help treat nausea and vomiting;
- Safe medications to help treat anxiety and depression in pregnancy;
- Availability of social workers, physicians, or other health care providers to assist with quit plans;
- Positive drug screening at delivery may prompt notification of child protective services, depending on state laws or health system policies.
MARIJUANA USE AND BREASTFEEDING
Uniform guidelines regarding the varied use of marijuana by breastfeeding mothers are difficult to create and cannot hope to cover all situations. The legality of possessing and using marijuana varies greatly from country to country; in the United States, there are increasing numbers of states where it is legal for "medicinal use" with a prescription, and a few states where it is legal for "recreational use," but under federal law, it remains illegal in all states. Therefore, basing recommendations on marijuana use and concurrent breastfeeding from a purely legal standpoint becomes inherently complex, problematic, and impossible to apply uniformly across all settings and jurisdictions. As laws shift and marijuana use becomes even more common in some areas, it becomes increasingly important to carefully weigh the risks of initiation and continuation of breastfeeding while using marijuana with the risks of not breastfeeding while also considering the wide range of occasional, to regular medical, to heavy exposure to marijuana. In addition to the potential legal risk, the health risks to the infant from the mother's marijuana use must be carefully considered. THC, the main compound in marijuana, is present in human milk up to eight times that of maternal plasma levels, and metabolites are found in infant feces, indicating that THC is absorbed and metabolized by the infant. It is rapidly distributed to the brain and adipose tissue and stored in fat tissues for weeks to months. It has a long half-life (25-57 hours) and stays positive in the urine for 2-3 weeks, making it impossible to determine who is an occasional versus a chronic user at the time of delivery by urine toxicology screening. Evidence regarding the effects of THC exposure on infant development via breastfeeding alone is sparse and conflicting, and there are no data evaluating neurodevelopmental outcomes beyond the age of 1 year in infants who are only exposed after birth. Also notable in this discussion of risk is that the potency of marijuana has been steadily increasing, from about 3% in the 1980s to 12% in 2012, so data from previous studies may no longer even be relevant. Additionally, current concern over marijuana use during lactation stems from possible infant sedation and maternal inability to safely care for her infant while directly under its influence; however, this remains a theoretical problem and has not been well established in the literature (40).
Marijuana can be passed to infants through their mother's breast milk. Marijuana may also affect the quality and quantity of breast milk that you make. Although no consistent effects have been noticed in infants exposed to marijuana through breast milk, the American Academy of Pediatrics (AAP) advises that breastfeeding mothers avoid using marijuana. Be sure to talk to your health care provider about all your choices for breastfeeding. There are insufficient data to evaluate the effects of marijuana use on infants during lactation and breastfeeding, and in the absence of such data, marijuana use is discouraged. Human and animal evidence examining the behavioral and neurobiological effects of exposure to cannabinoids during pregnancy and lactation shows that the endocannabinoid system plays a crucial role in the ontogeny of the central nervous system and its activation, during brain development. Cannabinoid exposure during critical periods of brain development can induce subtle and long-lasting neuro-functional alterations. Several preclinical studies highlight how even low to moderate doses during particular periods of brain development can have profound consequences for brain maturation, potentially leading to long-lasting alterations in cognitive functions and emotional behaviors. Exposure to second-hand marijuana smoke by infants has been associated with an independent two times possible risk of sudden infant death syndrome (SIDS) (III) - Quality of evidence [levels of evidence I, II-1, II-2, II-3, and III] is based on the U.S. Preventive Services Task Force Appendix A Task Force Ratings. And because breastfeeding reduces risk of SIDS, this needs to be additionally considered (42). Thus, careful risk / benefit evaluation to newborns who are substantially benefitted from breastfeeding and human milk, and as do their mothers, is needed for an individual case. A prenatal plan preparing the mother for parenting, breastfeeding, and substance abuse treatment should be formulated through individualized, patient-centered discussions with each woman. This care plan should include instruction in the consequences of relapse to drug or excessive alcohol use during lactation, as well as teaching regarding potential for donor milk, formula preparation, and bottle handling and cleaning should breastfeeding become contraindicated. In the perinatal period each mother-infant dyad should be carefully and individually counseled on breastfeeding prior to discharge from maternity care.
CHILD DEVELOPMENT
Marijuana is highly lipid soluble and crosses the placenta and the blood-brain barrier with ease, accumulating in fetal tissues, particularly the brain. In the adult central nervous system, THC interferes with the endocannabinoid singling system, responsible for modulating synaptic neurotransmitter release to regulate motor control, memory, and other brain functions. Components of the endocannabinoid system are present during embryonic central nervous system development as early as 16-22 days' gestation in humans (44). It is at this time that the neural plate and neural tube, the basic scaffold for the forebrain, midbrain, and hindbrain, are established. A large study conducted by the US National Birth Defects Prevention Center documented a significantly increased risk for anencephaly when the fetus is exposed to marijuana during the first month of gestation (45). This risk was isolated to the period when the neural tube is closing, 1-4 weeks after conception. The function of the endocannabinoid system during the pre-neuronal phase in humans has not been well delineated. However, a long line of research has demonstrated its important role in shaping neuronal circuitry in the developing fetus as well as modulating development of various neurotransmitter system, primarily the catecholaminergic and opioidergenic systems. Gestational exposure to exogenous cannabinoids, as found in marijuana, may target the cannabinoid receptor CB1, disrupting migration, differentiation, and synaptic communication in the developing neurotransmitter system (45).
There is also an evidence that intrauterine exposure to marijuana impairs dopamine D2 mRNA expression in the amygdala and in the nucleus accumbens at around 18-22 weeks' gestation (46). The resulting defective dopamine D2 signaling in these centers, which play a role in cognitive and emotional functioning, is consistent with the neuro-behavioral deficiencies that have been observed in newborns exposed to marijuana. These deficits primarily reflect impaired regulatory control: irritability, tremors, and poor habituation; difficulty with arousal and state regulation; and sleep disturbance. Although two studies found no neuro-behavioral differences between marijuana-exposed and non-exposed infants in the early neonatal period, it has been postulated that these two studies differed from the others because of sociocultural differences as well as the varying statistical treatments of the different confounding factors (47).
Numerous studies have documented neuro-developmental deficits in older children, adolescents, and young adults who were prenatally exposed to marijuana (48). At 6 years of age, prenatal marijuana exposure was linked to lower verbal reasoning scores and deficits in composite, short-term memory, and quantitative intelligence scores. In this same cohort at 10 year of age, negative effects of prenatal marijuana exposure had a significant impact on design memory and screening index scores the Wide Range Assessment of Memory and Learning (49), and the exposed children had lower test scores on school achievement. In addition, by age 10 years, prenatal marijuana exposure was significantly related to increased hyperactivity, impulsivity, and inattention problems as well as significantly increased rates of child depressive symptoms.
Child depressive symptoms and attention problems in these children at age 10 significantly predicted delinquency at 14 years of age. The prenatal exposure to marijuana exposure is related to a significantly increased rate of difficulties with executive functioning, an aspect of regulatory control that is key to learning and to managing behavior. A study of functional magnetic resonance imaging (MRI) in a group of 18-22 year old young adults who had been prenatally exposed to marijuana revealed altered neural functioning that impacted short-term memory (50). Further animal and human studies are needed, especially studies that can overcome the common limitations found in the majority of studies that investigate teratogenic agents in humans, specifically the inability to conduct randomized, controlled, prospective studies and the reliance on retrospective self-report regarding amounts and patterns of marijuana use.
PATIENT EDUCATION
If a patient is prescribed a cannabinoid or medical cannabis, he or she should be advised of possible memory impairment and instructed to report any mental or behavioral changes. In addition, operating a vehicle or heavy machinery is not recommended after having taken the drug, and patients should limit or abstain from alcohol. All patients should be monitored for outcomes, similar to the processes used for opioid follow-up monitoring. Any concomitant medications and drug interactions should also be monitored. For example, there is little evidence of clinically significant CYP450 interactions, but co-administration may potentiate somnolence. Side effects should be noted and reported; however, it is important to note that tolerance may develop over time to side effects of mild-to-moderate severity. Smoking or vaporization should cease if a patient begins experiencing disorientation, dizziness, ataxia, agitation, anxiety, tachycardia and orthostatic hypotension, depression, hallucinations, or psychosis (43).
For patients who are not proficient in English, it is important that information regarding the benefits and risks associated with the use of medical marijuana and other cannabinoids be provided in their native language, if possible. When there is an obvious disconnect in the communication process between the practitioner and patient due to the patient's lack of proficiency in the English language, an interpreter is required. Interpreters can be a valuable resource to help bridge the communication and cultural gap between patients and practitioners. Interpreters are more than passive agents who translate and transmit information back and forth from party to party. When they are enlisted and treated as part of the interdisciplinary clinical team, they serve as cultural brokers who ultimately enhance the clinical encounter. In any case in which information regarding treatment options and medication / treatment measures are being provided, the use of an interpreter should be considered. Print materials are also available in many languages, and these should be offered whenever necessary.
POLICY IMPLICATIONS
The number of physicians who are prescribing marijuana to pregnant women across the various states is unknown, but professional organizations have recognized the need to address the issue. The American Medical Association (AMA) announced in 2015 that it would advocate for regulations and pregnancy warning labels on medical and recreational marijuana, and in July 2015 the Committee on Obstetric Practice of the American College of Obstetricians and Gynecologists (ACOG) published a policy statement that discouraged obstetricians and gynecologists from "prescribing or suggesting the use of marijuana for medicinal purposes during preconception, pregnancy and lactation" (51). From a public health perspective, state departments of health, in collaboration with state licensing boards, should take several steps to educate and inform the public and professionals on the possible impact of marijuana's use during pregnancy and to discourage such use including the following (52):
- Medical marijuana legislation should include public, professional, and legislative education about the impact of marijuana on pregnancy and child outcome;
- Informational materials should be available at all sites that prescribe or sell marijuana, and a government warning label, similar to alcohol, regarding marijuana use and pregnancy should be posted;
- Physicians who plan to write marijuana prescriptions should be required to obtain continuing medical education credits that address marijuana and pregnancy;
- Guidelines for physicians writing marijuana prescriptions should be developed, including asking all women of child-bearing about the possibility of a current pregnancy and offering a pregnancy test to all women of child-bearing age prior to giving a prescription for marijuana;
- Long-term controlled evaluations of infants exposed to marijuana via human milk, to include infants and later neurodevelopmental outcomes, including those exposed to marijuana in a controlled manner, such as with legalized medical marijuana.
From a public health perspective, at the very least, we must acknowledge that marijuana's use during pregnancy has potential risks, and we need to incorporate guidelines into the new and emerging marijuana laws that recognize and communicate that risk. Marijuana use is fast fading from the legal agenda, but its use, especially during pregnancy, remains a public health issue.
Summary
Marijuana is the most common recreational drug used in pregnancy. In the obstetric population, the reported prevalence ranges from 2% to 27% depending on population studied, definition of use, and method of detection. This is likely under-estimated because marijuana is often under-reported. Despite the fact that hundreds of thousands of women use marijuana in pregnancy, little is known about the effects of marijuana on neonatal outcomes. It is biologically plausible that marijuana use during pregnancy could affect the fetus. Delta9-tetrahydrocannabinol (THC) readily crosses the placenta and can be detected in the adult body for 30 days. In addition, when smoked, marijuana has been shown to lead to five-fold higher serum carbon monoxide levels compared to tobacco, potentially leading to impaired maternal respiratory and gas exchange physiology and subsequent harmful effects on the fetus. Because marijuana is neither regulated nor evaluated by the FDA in the United States, there are no approved indications, contraindications, safety precautions, or recommendations regarding its use during pregnancy and lactation. Likewise, there are no standardized formulations, dosages, or delivery systems. Smoking, the most common route of administration of THC, cannot be medically condoned during pregnancy and lactation. Therefore, healthcare providers should be discouraged from prescribing or suggesting the use of marijuana for medicinal purposes during preconception, pregnancy, and lactation. Rather pregnant women or women contemplating pregnancy should be encouraged to discontinue use of marijuana for medicinal purposes in favor of an alternative therapy for which there are better pregnancy-specific safety data. High-quality studies regarding the effects of marijuana and other cannabis products on pregnancy and lactation are needed.
Medical marijuana has become a hot topic in health care. Initiatives to either legalize or prohibit marijuana use for medical purposes are being legalized by politicians or presented to voters in numerous municipalities. The preponderance of information on this subject seems to come from highly visible individuals or groups who either vehemently oppose or passionately advocate legal access to medical cannabis. What is most needed is a comprehensive presentation of the scientific facts from a dispassionate, evidence-based perspective. This course has reviewed the body of research on medical cannabis to provide the most current information on potential indications, pharmacology and mechanism of action, acute and chronic side effects, and contraindications for medicinal cannabis. A clear understanding of the potential uses of cannabinoids in the treatment of various medical conditions will benefit patients and healthcare providers alike.
Although there is much to learn yet about the effects of prenatal marijuana use on pregnancy and child outcome, there is enough evidence to suggest that marijuana contrary to popular perception, is not a harmless drug, especially when used during pregnancy. Consequently, the public health system has a responsibility to educate physicians and the public health about the impact of marijuana on pregnancy and to discourage the use of medical marijuana by pregnant women or women considering pregnancy. In the United States, women who have established breastfeeding and subsequently relapse to illegal drug use are counseled not to breastfeed, even if milk is discarded during the time period surrounding relapse. There are no known pharmacokinetic data to establish the presence and/or concentrations of most illicit substances and/or their metabolites in human milk and effects on the infant, and this research is unlikely to occur given the ethical dilemmas it presents. The lack of pharmacokinetic data for most drugs of abuse in recently postpartum women with substance use disorders precludes the establishment of a "safe" interval after use when breastfeeding can be reestablished for individual drugs of abuse. Additionally, women using illicit substances in the postnatal period may exhibit impaired judgment and secondary behavioral changes that may interfere with the ability of the mother to care for her infant or to breastfeed adequately.
SUGGESTED READING
- World Health Organization
The Health and Social Effects of Nonmedical Cannabis Use
http://apps.who.int/iris/bitstream/10665/251056/1/9789241510240-eng.pdf?ua=1 - NIH, U.S. National Library of Medicine
Marijuana and Pregnancy
https://medlineplus.gov/marijuana.html
References
- U.S. Drug Enforcement Agency. In the Matter of Lyle E. Craker, Ph.D. Docket No. 05-16. Opinions and Recommended Rulings, Administrative Law Judge. Available at https://www.aclu.org/files/images/asset_upload_file116_28341.pdf Last accessed March 20, 2016
- McPartland JM, Guy GW. The evolution of Cannabis and co-evolution with the cannabinoid receptor: a hypothesis. In: Guy GW, Whittle BA, Robson PJ (eds). The Medicinal Use of Cannabis and Cannabinoids. London: Pharmaceutical Press; 2004; 71-101
- National Institute on Drug Abuse. Nationwide trends. Available at: https://www.drugabuse.gov/publications/research-reports/marijuana/letter-director Accessed on March 22, 2016
- Saurel-Cubizolles MJ, Prunet C, Blondel B. Cannabis use during pregnancy in France in 2010. BJOG 2014;121:971-977
- Monte AA, Zane RD, Heard KJ. The implications of marijuana legalization in Colorado. JAMA 2015;13:241-242
- Colorado Department of Revenue. Colorado Marijuana Tax Data. Available at: https://www.colorado.gov/pacific/revenue/colorado-marijuana-tax-data Accessed on 1 April 2016
- Light M, Orens A, Lewandowski B, et al. Market size and demand for marijuana in Colorado, prepared for the Colorado Department of Revenue. 2014. Available at: https://www.colorado.gov Accessed on January 17, 2017
- Bostwick JM, Reisfield GM, DuPont RL. Clinical decisions: medicinal use of marijuana. N Engl J Med 2013;368(9):866-868
- Lynne-Landsman SD, Livingston MD, Wagenaar AC. Effects of state medical marijuana laws on adolescent marijuana use. Am J Public Health 2013;103(8):1500-1506
- Tennes K, Avitable N, Blackard C, et al. Marijuana: prenatal and postnatal exposure in the human. NIDA Res Monogr 1985;59:48-60
- Moore DG, Turner JD, Parrott AC, et al. During pregnancy, recreational drug-using women stop taking ecstasy (3,4-methylenedioxy-N-methyl-amphetamine) and reduce alcohol consumption, but continue to smoke tobacco and cannabis: initial findings from the Development and Infancy Study. J Psychopharmacol 2010;24:1403-1410
- Pearson G, Shiner M. Rethinking the generation gap: Attitudes to illicit drugs among young people and adults. Crim Justice 2002;2:15
- Mallat A, Teixeira-Clerc F, Deveaux V, et al. The endocannabinoid system as a key mediator during liver diseases: new insights and therapeutic openings. British Journal of Pharmacology 2011;163(7):1432-1440
- Russo EB. Cannabinoids in the management of difficult to treat pain. Therapeutics and Clinical Risk Management 2008;4(1):245-259
- Pertwee RG, Howlett AC, Abood ME, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB and CB. Pharmacol Rev 2010;62(4):588-631
- Patel KD, Davison JS, Quentin J, Pittman OJ, Sharkey KA. Cannabinoid CB2 receptors in health and disease. Curr Med Chem 2010;17(14):1394-14
- Hill AJ, Williams CM, Whalley BJ, Stephens GJ. Phytocannabinoids as novel therapeutic agents in CNS disorders Pharmacol Ther 2012;133(1):79-97
- Mura P, Kintz P, Dumestre V, et al. THC can be detected in brain while absent in blood. J Anal Toxicol 2005;29(8):842-843
- Aggarwal SK. Cannabinergic pain medicine: a concise clinical primer and survey of randomized-controlled trial results. Clin J Pain 2013;29(2):162-71
- Grotenhermen F. Pharmacology of cannabinoids. Neuro Endocrinol Lett 2004;25(1-2):14-23
- Mechoulam R. Cannabis: a valuable drug that deserves better treatment. Mayo Clin Proc 2012;87(2):107-109
- Grant I, Atkinson JH, Gouaux B, Wilsey B. Medical marijuana: clearing away the smoke. Open Neurol 2012;6:18-25
- Crippa JAS, Derenusson GN, Chagas MHN, et al. Pharmacological interventions in the treatment of the acute effects of cannabis: a systematic review of literature. Harm Reduction Journal 2012;9:7
- Chen R, Zhang J, Fan N, et al. Delta9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell 2013;155(5):1154-1165
- Leung L. Cannabis and its derivatives: review of medical use. J Am Board Fam Med 2011;24(4):452-462
- Melamede R. Cannabis and tobacco smoke are not equally carcinogenic. Harm Reduction Journal 2005;2:21
- Tan WC, Lo C, Jong A, et al. Marijuana and chronic obstructive lung disease: a population-based study. CMAJ 2009;180(8):814-820
- Chao C, Jacobson LP, Tashkin D, et al. Recreational drug use and T lymphocyte subpopulations in HIV-uninfected and HIV-infected men. Drug Alcohol Depend 2008;94(1-3):165-171
- Schoeler T, Bhattacharyya S. The effect of cannabis use on memory function: an update. Substance Abuse and Rehabilitation 2013;4:11-27
- Decoster J, van Os J, Kenis G, et al. Age at onset of psychotic disorder: cannabis, BDNF Val66Met, and sex-specific models of gene-environment interaction. Am J Med Genet B Neuropsychiatr Genet 2011;156B(3):363-369
- Wang GS, Roosevelt G, Heard K. Pediatric marijuana exposures in a medical marijuana state. JAMA Pediatr 2013;167(7):630-633
- Gorelick DA, Levin KH, Copersino ML, et al. Diagnostic criteria for cannabis withdrawal syndrome. Drug Alcohol Depend 2012;123(1-3):141-147
- Lynne-Landsman SD, Livingston MD, Wagenaar AC. Effects of state medical marijuana laws on adolescent marijuana use. .Am J Public Health 2013;103(8):1500-1506
- Compassionate Care New York. Medical Marijuana in New York: Safeguards in A.6357-A/S.4406-A. Available at: http://www.compassionatecareny.org/wp-content/uploads/MMJ-Safegaurds-7.8.13.pdf?092a61 Last accessed on 12 January 2017
- Hazekamp, A, Heerdink ER. The prevalence and incidence of medicinal cannabis on prescription in the Netherlands. Eur J Clin Pharmacol 2013;69(8):1575-1580
- Office of Medicinal Cannabis in the Netherlands. Grounds for Use. Available at https://www.cannabisbureau.nl/English Last accessed 30 January 2017
- Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol 2016;215:506.e1-7
- Conner SN, Bedell V, Lipsey K, et al. Maternal marijuana use and adverse neonatal outcomes. Obstet Gynecol 2016;128:713-723
- Holland CL, Rubio D, Rodriguez KL, et al. Obstetric health care providers' counseling response to pregnant patient disclosures of marijuana use. Obstet Gynecol 2016;127:681-687
- Pew Research Center. Majority now supports legalizing marijuana. 2013. Available at: http://www.people-press.org/2013/04/04/majority-now-supports-legalizing-marijuana/ Retrieved 3 February 2017
- Reece-Stremtan S, Marinelli KA, and The Academy of Breastfeeding Medicine. ABM Clinical Protocol # 21; Guidelines for breastfeeding and substance use or substance use disorder, Revised 2015. Available @ http://online.liebertpub.com/doi/pdf/10.1089/bfm.2015.9992 Retrieved 6 February 2017
- Klonoff-Cohen H, Lam-Kruglick P. Maternal and paternal recreational drug use and sudden infant death syndrome. Arch Pediatr Adolesc Med 2001;155:765-770
- Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain: consensus statement and guidelines from the Canadian Pain Society. Pain Res Manage 2007;12(1):13-21
- Trezza V, Cuoma V, Louk J, et al. Cannabis and the developing brain: insights from behavior. Eur J Pharmacol 2008;585:441-452
- vanGelder MM, Reefhuis J, Caton AR, et al. Endocannabinoid illicit drug use and the risk of congenital malformations. Epidemiology 2008;20:60-66
- Wang X, Dow-Edwards D, Anderson V, et al. In utero marijuana exposure associated with abdominal amygdala dopamine D2 gene expression in the human fetus. Biol Psychiatry 2004;56:909-915
- Maraes Barros MC, Guinsburg R, Araujo Peres C. exposure to marijuana during pregnancy alters neuro-behavior in the early neonatal period. J Pediatr 2006;149:781-784
- Day NL, Leech SL, Goldschmidt L. The effects of prenatal marijuana exposure on delinquent behaviors are mediated by measures of neuro-cognitive functioning. Neurotoxicol Teratol 2011;33:1-11
- Jastak S, Wilkinson GS. Manual for the Wide Range Achievement Test, revised. Wilmington (DE): Jastak Associates; 2008.
- Smith AM, Longo CA, Fried PA, et al. Effects of prenatal marijuana on visuospatial working memory: and fMRI study in young adults. Neurotoxicol Teratol 2006;28:286-295
- American College of Obstetricians and Gynecologists. Marijuana during pregnancy and lactation. ACOG Committee Opinion no. 637. Obstet Gynecol 2015;126:234-238
- Chasnoff IJ. Medical marijuana laws and pregnancy: implications for public health policy. AJOG 2017;216(1):27-30
Publicado: 22 March 2017
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


