Неонатальная стрептококковая инфекция группы B
Бюллетень WHEC Практика и клинической управления для медицинских работников. Образование гранта, предоставленного здоровья женщин и образовательный центр (WHEC).
Group B streptococcal (GBS) infection remains the most common cause of neonatal early-onset sepsis and a significant cause of late-onset sepsis among young infants. Administration of intrapartum antibiotic prophylaxis is the only currently available effective strategy for the prevention of perinatal GBS early-onset-disease, and there is no effective approach for the prevention of late-onset disease. The Women’s Health and Education Center (WHEC) reaffirms the use of universal antenatal microbiologic-based testing for the detection of maternal GBS colonization to facilitate appropriate administration of intrapartum antibiotic prophylaxis.
The purpose of this document is to provide healthcare providers, with updated information regarding the epidemiology of GBS disease, as well current recommendations for the evaluation of newborn infants at risk for GBS disease, and for treatment of those with confirmed GBS infection. This review addresses the epidemiology, microbiology, disease pathogenesis, and management strategies for neonatal early- and late-onset GBS infection. Maternal management is discussed in the “Prevention of Group B Streptococcal Infections in Newborn.”
Incidence
Healthcare providers should recognize that GBS early-onset-disease can occur among term infants born to mothers who have screened negative for GBS. Authors of one single-center study with policies mandating universal screening-based GBS intrapartum antibiotic prophylaxis found that over an 8-year period, 17 GBS early-onset cases occurred among term infants and 14 of 17 (82.4%) of the mothers has screened negative for GBS (1). Multistate Active Bacterial Core surveillance’s (ABC’s) data in 2003-2004 identified 189 cases of GBS early-onset-disease among term infants and determined that 116 of 189 (61.4%) occurred among infants born to women who screened negative for GBS (2). GBS early-onset disease may occur in infants of mothers who screened negative for GBS because of changes in maternal colonization status during the interval from screening to presentation for delivery or because of an incorrect technique in obtaining vaginal-rectal screening cultures or in laboratory processing.
Prevention of Group B Streptococcal Infections in Newborns; Antepartum and Intrapartum Management
http://www.womenshealthsection.com/content/obs/obs037.php3
Current Epidemiology of Neonatal GBS Infection
Early-Onset GBS Infection
GBS early-onset-disease is defined as isolation of group B Streptococcus organisms from blood, cerebrospinal fluid (CSF), or another normally sterile site from birth through 6 days of age (5). Meningitis was diagnosed in 9.5% of infants with GBS early-onset-disease. CSF culture-positive GBS early-onset-disease occurred in the absence of bacteremia in 9.1% of early-onset meningitis cases (incidence: approximately 2.5 cases per 1 million live births). Infants born at <37 weeks’ gestation account for 28% of all GBS cases; approximately 15% of cases occur among preterm infants with very low birth weight (<1,500 g) (4,6). Overall GBS infection accounts for approximately 45% of all cases of culture-confirmed early-onset sepsis among term infants and approximately 25% of all early-onset sepsis cases occur among infants with very low birth weight. Death attributable to GBS early-onset-disease occurs primarily among preterm infants: the current case fatality ratio is 2.1% among term infants and 19.2% among those born at <37 weeks’ gestation (6).
Newborn infants readmitted to the hospital within the first week after hospital discharge with culture-confirmed infection attributable to any bacteria, in this study, was approximately 5 cases per 100,000 live birth (7). Globally, outside the United States, an estimated 200,000 GBS cases of GBS early-onset-disease occurred in 2015. Stillbirth, GBS early-onset-disease, and late-onset GBS cases combined contribute to an estimated 150,000 fetal and neonatal deaths throughout the world, with the largest concentration of GBS perinatal deaths occurring in Africa (8).
Late-Onset GBS Disease
GBS late-onset-disease is defined as isolation of GBS from a normally sterile site from 7 to 89 days of age (5). Rarely, very-late-onset GBS disease may occur after 3 months of age, primarily among infants born very preterm or infants with immunodeficiency syndromes (9). GBS late-onset-disease rates have not changed with widespread use of intrapartum antibiotic prophylaxis. GBS late-onset-disease incidence was stable over the 2006 – 2015 ABC’s study period, with an average incidence of 0.31 cases per 1,000 live births (4). The median age at presentation with GBS late-onset-disease was 34 days (interquartile range: 20-49 days). Bacteremia was identified in approximately 93% of GBS late-onset-disease, and bacteremia without focus was the most common form of disease.
GBS were isolated from CSF in 20.7% of cases, and meningitis was diagnosed in 31.4% of cases. Cultures of bone and joint and peritoneal fluid yielded GBS in 1.8% of cases (10). CSF culture-positive GBS late-onset-disease occurred in the absence of bacteremia in approximately 20% of late-onset meningitis cases (incidence: 1.9 cases per 100,000 live births). Infants born at <37 weeks’ gestation approximately 42% of all GBS late-onset-disease cases, and death attributable to GBS late-onset-disease occurs in preterm infants at roughly twice the rate of term infants (7.8% vs 3.4%, respectively) (10). GBS late-onset-disease complicated by meningitis has a higher patient fatality rate than with other syndromes.
Pathogenesis and Risk Factors
GBS Virulence
Bacterial factors promote invasive GBS infection. GBS are characterized by immunologically distinct surface polysaccharide capsules that define 10 serotypes (type I, Ia, and II-IX). Worldwide, serotypes I-V account for 98% of carriage and 97% of infant invasive strains; serotype III accounts for approximately 25% of colonizing strains and approximately 62% of invasive infant strains, with regional variation (11).
Surveillance data in the United States for invasive strains from 2006 to 2015 revealed that 93.1% of GBS early-onset-disease cases were attributable to serotypes Ia (27.3%), III (27.3%), II (15.6%), V (14.2%), and Ib (8.8%); the proportion attributable to emerging serotype IV ranged from 3.4% to 11.3% over the study period (4). Serotype III accounted for approximately 56.2% of 1,387 GBS late-onset-disease cases during 2006 to 2015, with serotypes Ia (20%), V (8.3%), IV (6.2%), and Ib (6.1%) making up most of the remaining serotypes.
The capsular polysaccharide of all GBS serotypes resists complement deposition and inhibits opsonophagocytosis. Maternally derived, serotype-specific antibody to maternal colonizing GBS isolates is protective against newborn infection (12). GBS express multiple additional virulence factors, including surface proteins such as the α and β C-proteins that promote adherence and immune evasion, pore-forming toxins such as β-hemolysin and CAMP factor, and secreted proteases such as the C5a peptidase that cleaves complement. Strains vary in their expression of virulence factors, many of which are highly regulated by 2-component regulatory systems. The hypervirulent serotype III multi-locus sequence type 17 (ST17), for example, is commonly found in cases of GBS meningitis (13).
Risk Assessment of Early-Onset GBS Infection
Because the pathogenesis of GBS early-onset-disease begins with vertical transmission of GBS from mother to fetus and newborn infant, the strongest predictor of GBS early-onset-disease is maternal GBS early-onset-disease, such as the virulence of maternal colonizing isolate and the presence of maternal serotype-specific protective antibody, cannot be known to the physician at the time of neonatal risk assessment. The newborn infant’s condition at birth and evolving condition over the first 12 to 24 hours after birth are strong predictors of early-onset infection attributable to any pathogen (14). In summary, at this time, evidence supports the following:
- The definitive diagnosis of intraamniotic infection is made by amniotic fluid Gram-stain and/or culture or by placental histopathologic testing. Suspected intraamniotic infection is defined as a single maternal intrapartum temperature >39OC or maternal temperature of 38OC to 38.9OC in combination with 1 or more of maternal leukocytosis, purulent cervical drainage, or fetal tachycardia.
- The routine measurement of complete blood cell counts or inflammatory markers such as C-reactive protein alone in newborn infants to determine risk of GBS early-onset-disease is not justified given the poor test performance of these in predicting what is currently a low-incidence disease (15).
- Newborn clinical illness consisting of abnormal vital signs (e.g., tachycardia, tachypnea, and/or temperature instability), supplemental oxygen requirement and/or need for continuous positive airway pressure, mechanical ventilation, or blood pressure support can be used to predict early-onset infection (14). There is no evidence that hypoglycemia occurring in isolation in otherwise well-appearing infants is a risk factor for GBS early-onset-disease of early-onset sepsis. A newborn’s clinical condition often evolves in the hours after birth, and physicians must exercise judgment to distinguish transitional instability from sighs of clinical illness.
Management Strategies
There are three Current Approaches to Risk Assessment among infants born at >35 Weeks’ Gestation
1. Categorical Risk Assessment: It uses risk factor threshold values to identify infants at increased risk of GBS early-onset-disease (10). See figure 1 A below.
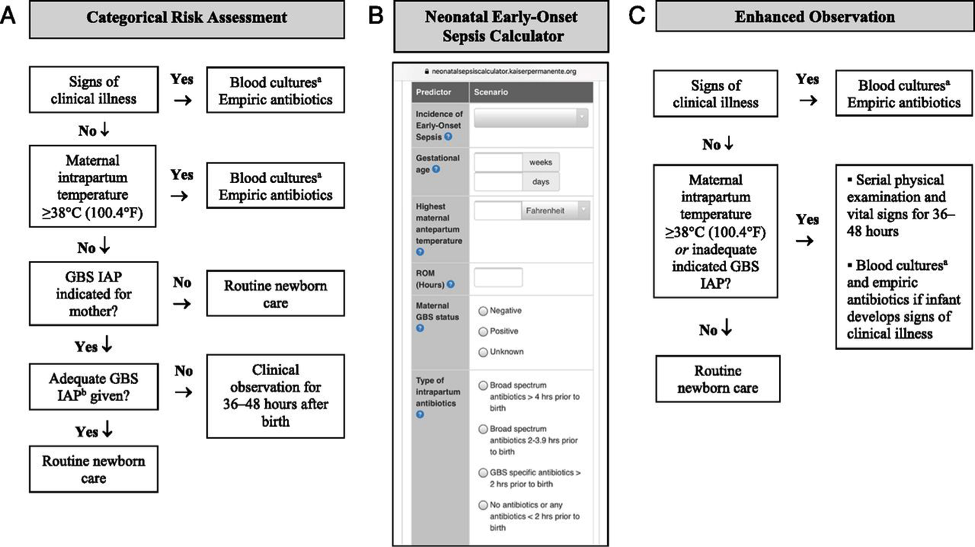
Figure 1. Options for early-onset-sepsis risk assessment among infants born >35 weeks’ gestation. A: categorical risk assessment; B: Neonatal Early-Onset Sepsis calculator; C: Enhanced observation. Abbreviations: GBS (group B streptococcus); IAP (intrapartum antibiotic prophylaxis); CSF (cerebro-spinal fluid)
a Consider lumbar puncture and CSF culture before initiation of empiric antibiotics for infants who are at the highest risk of infection, especially those with critical illness. Lumbar puncture should not be performed if the infant’s clinical condition would be compromised, and antibiotics should be administered promptly and not deferred because of procedure delays.
b Adequate GBS intrapartum prophylaxis is defined as the administration of penicillin G, ampicillin, or cefazolin >4 hours before delivery.
The administration of penicillin G, ampicillin, or cefazolin >4 hours before delivery is considered adequate GBS intrapartum antibiotic prophylaxis; other antibiotics or other durations of treatment <4 hours are considered inadequate when using this approach. Substantial data have been reported on the impact of using categorical risk factors to manage the risk of GBS early-onset-disease (16). However, the risk is highly variable among the newborn infants recommended to receive empirical treatment in this approach, ranging from slightly lower than the baseline population risk to significantly higher, depending on the gestational age, duration of rupture of membranes, and timing and content of administrated intrapartum antibiotics. Consequently, a limitation of this approach is that categorical management will result in empirical treatment of many relatively low-risk newborn infants.
2. Multivariate risk assessment (the Neonatal Early-Onset Sepsis Calculator): It integrates the individual infant’s combination of risk factors and the newborn infant’s clinical condition to estimate an individual infant’s risk of early-onset sepsis, including GBS early-onset-disease. Predictive models based on gestational age at birth, highest maternal intrapartum temperature, maternal GBS colonization status, duration of rupture of membranes, and type and duration of intrapartum antibiotic therapies have been developed and validated. These models are available as a Web-based Neonatal Early-Onset Sepsis Calculator (See figure 1B above) or available @: https://neonatalsepsiscalculator.kaiserpermanente.org/
When using models, only penicillin, ampicillin, or cefazolin should be considered as “GBS-specific antibiotics.” The administration of clindamycin or vancomycin alone for intrapartum antibiotic prophylaxis for any duration is currently recommended to be entered as “no antibiotics.” Because the models were developed to predict risk of all bacterial causes of early-onset sepsis (and not just GBS early-onset-disease), and because these models account for other antibiotic types and indications for intrapartum antibiotic administration, “GBS specific antibiotics >2 hours prior to birth” is 1 of calculator variables. The 2-hour timing is used because multiple factors in addition to GBS intrapartum prophylaxis are considered when using the multivariate models in the Neonatal Early-Onset Sepsis Calculator. Used in this manner, threshold risk estimates prompting enhanced clinical observation or blood culture and empirical antibiotic therapy have been prospectively validated in large newborn cohorts (17).
3. Risk assessment based on newborn clinical condition: A final approach to GBS early-onset-disease risk assessment is to rely on clinical signs of illness to identify infants who may be at increased risk of infection. Among term infants, good clinical condition at birth is associated with an approximately 60% to 70% reduction in risk for early-onset infection (14). Under this approach, infants who appear ill at birth and whose who develop signs of illness over the first 48 hours after birth are treated empirically with antibiotics (18).
Physicians and families must understand that the identification of initially well-appearing infants who develop clinical illness is not failure of care, but rather an anticipation outcome of this approach to GBS early-onset-disease risk management.
Infants Born at <34 Weeks’ Gestation, The Optimal Approach to Risk Assessment
Clinician may adopt one of the following strategies to develop institutional approaches best suited to their local resources and structure of care.
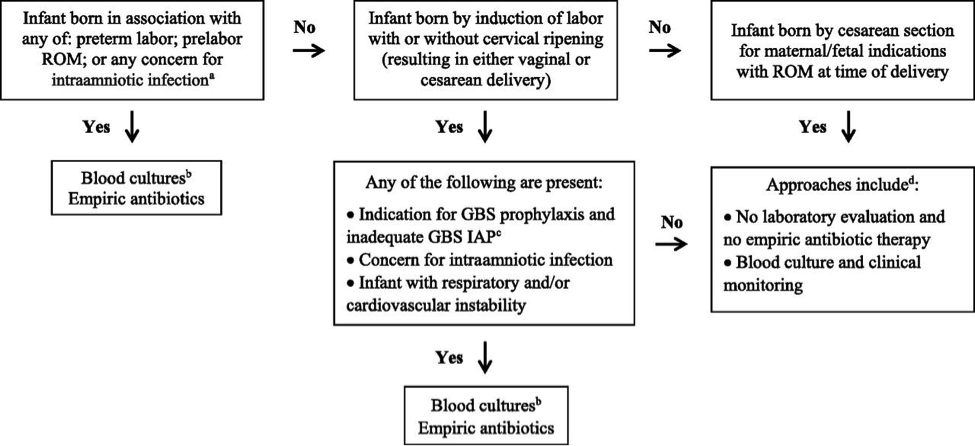
Figure 2. Early-onset sepsis risk assessment among infants born <34 weeks’ gestation. Abbreviations: IAP (intrapartum antibiotic prophylaxis); GBS (group B streptococcus); ROM (rupture of membranes); CSF (cerebro-spinal fluid);
a Intraamniotic infection should be considered when a pregnant woman presents with unexplained decreased fetal movement and/or there is sudden and unexplained poor fetal testing.
b Lumbar puncture and CSF culture should be performed before initiation of empiric antibiotics for infants who are at the highest risk of infection unless the procedure would compromise the infant’s clinical condition. Antibiotics should be administered promptly and not deferred because of procedural delays.
c Adequate GBS intrapartum antibiotics prophylaxis is defined as the administration of penicillin G, ampicillin, or cefazolin >4 hours before delivery.
d For infants who do not improve after initial stabilization and/or those who have severe systemic instability, the administration of empiric antibiotics may be reasonable but is not mandatory.
Preterm infants at highest risk for early-onset sepsis: Infants born preterm because of cervical insufficiency, preterm labor, prelabor premature rupture of membranes, intraamniotic infection, and/or acute or otherwise unexplained onset of non-reassuring fetal status are at the highest risk of early-onset sepsis and GBS early-onset-disease. The administration of GBS intrapartum antibiotic prophylaxis may decrease the risk of infection among these infants, but the most reasonable approach to these infants is to obtain a blood culture and start empirical antibiotic treatment. A lumbar puncture for culture and analysis of CSF should be considered in clinically ill infants when there is a high suspicion for GBS early-onset-disease unless the procedure will compromise the neonate’s clinical condition.
Preterm infants at lower risk for early-onset sepsis: Preterm infants at lowest risk for all early-onset sepsis and for GBS early-onset-disease are those born under circumstances that include all of these criteria (19):
- Maternal and/or fetal indications for preterm birth (such as maternal preeclampsia or other non-infectious medical illness, placental insufficiency, or fetal growth restriction),
- Birth by cesarean delivery; and
- Absence of labor, attempts to induce labor, or any rupture of membranes before delivery.
Acceptable initial approaches of these infants include no laboratory evaluation and no empirical antibiotic therapy or blood culture and clinical monitoring. For infants who do not improve after initial stabilization and/or those who have severe systemic instability, the administration of empirical antibiotics may be reasonable but is not mandatory.
Infants delivered for maternal and/or fetal indications but who are ultimately born by vaginal or cesarean delivery after efforts to induce labor and/or with rupture of membranes before delivery are subject to factors associated with the pathogenesis of GBS early-onset-disease. If the mother has an indication for GBS intrapartum antibiotic prophylaxis and adequate intrapartum antibiotic prophylaxis (penicillin, ampicillin, or cefazolin >4hours before delivery) is not given or if any other concern of infection arises during the process of delivery, the infant should be managed as recommended above for preterm infants at higher risk for GBS early-onset-disease. Otherwise, an acceptable approach to these infants is close observation for those infants who are well appearing at birth and to obtain a blood culture and to initiate antibiotic therapy for infants with respiratory and/or cardiovascular instability after birth.
Clinical Presentation
The initial signs of sepsis may be subtle, and may include temperature instability, tachycardia, poor peripheral perfusion and respiratory distress. Because the progression of invasive disease is very rapid, any infant with clinical signs suggestive of infection should be treated immediately following a prompt full diagnostic evaluation; delay between presentation and therapy increases the risk of a poor outcome (20). There is no clear distinction in the clinical signs present when the infant has GBS sepsis compared with any other invasive organism.
Neither the maternal screening history nor intrapartum exposure to antibiotics should affect the approach to the management of the infant with clinical signs of sepsis. Therefore, prospective therapy, while waiting culture results, should cover the most common bacteria: GBS, other streptococci, Escherichia coli, other Gram-negative organisms and Listeria monocytogenes. An infant with signs of sepsis does not require confirmatory tests other than obtaining cultures before commencing therapy, because no other tests have an adequately high negative predictive value to avoid therapy. In particular, a normal white blood count (WBC) or differential should not prevent treatment in such an infant because the negative likelihood ratio of a normal complete blood count (CBC) is approximately 0.7 (21).
Limited diagnostic evaluation: It consists of CBC, and observation of vital signs every 4 hours for a period of 24 hours. The newborn can be cared for and observed in the mother’s postpartum room. If the CBC shows a low total WBC of less than 5.0X109/L, then the risk of sepsis is substantially increased and a full diagnostic evaluation an initiation of therapy would usually be indicated.
Full diagnostic evaluation: It consists of a CBC, blood culture and lumbar puncture (LP); a chest x-ray should be obtained if respiratory difficulties are present. LP can be deferred in unstable infants and performed later to ascertain the presence of hypoglycorrhachia or pleocytosis. Infants whose only sign of sepsis is respiratory distress may also be considered for deferment of LP if close follow-up can be ensured.
Empirical and Definitive Treatment
Table 1 below shows the current recommended intravenous (IV) antibiotic regimens for confirmed early- and late-onset GBS bacteremia and meningitis (5,22).
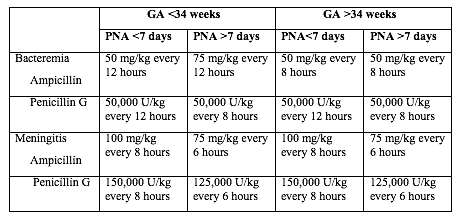
Table 1. Antibacterial drugs for neonates (<28 postnatal days of age). Abbreviation: GA (gestational age); PNA (postnatal age)
The length of antibiotic treatment is generally 10 days for bacteremia without focus and 14 days for uncomplicated meningitis; antibiotic should be given IV for the entire course. Longer therapy is used when there is a prolonged or complicated course. Some experts recommend a second LP for CSF culture 24 to 48 hours after the start of antibiotics. Additional LP and intracranial imaging are advised if there is not resolution of CSF infection, if neurologic abnormalities persist, or if focal deficits develop. Osteoarticular infection should be treated for 3 to 4 weeks and ventriculitis should be treated for at least 4 weeks. Consultation with a pediatric infectious disease specialist should be considered for meningitis and for cases with site-specific infection. Audiology testing and ongoing audiologic monitoring, if indicated, should be arranged before discharge.
Recurrent GBS Disease: Recurrent neonatal and young infant GBS disease can occur after completed appropriate treatment of the primary infection (23). In this study, during 2011 to 2015 the recurrence rate was 2.8% of cases of neonatal GBS infection (24). Recurrent cases were identified 3 to 54 days after completion of the therapy for the first course. Recurrent cases are generally caused by the same GBS serotype that caused the primary infection, and persistent mucosal colonization and poor neonatal antibody responses to the first infection likely contribute to the pathogenesis or recurrent infection. it is not preventable by extension of recommended antibiotic courses nor by the addition of rifampin to eradicate mucosal colonization (25).
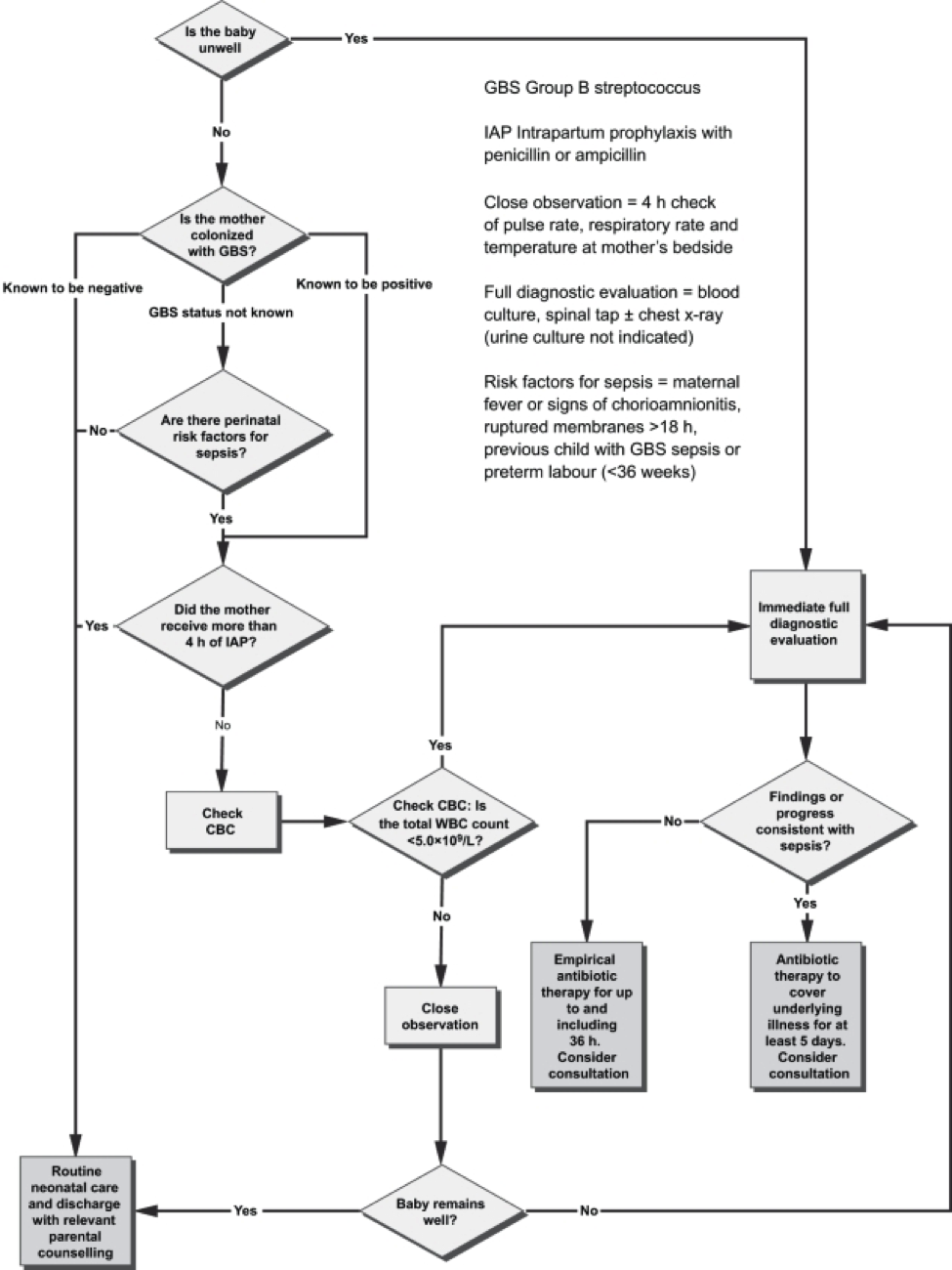
Figure 3. Algorithm for the management of newborn babies who may be at risk for neonatal sepsis. Source: Canadian Paediatric Society, 2007.
The mother who delivers at less than 37 weeks will often not have results of antenatal GBS screening available. In such a case, the infants has a ‘risk factor’ (prematurity) for invasive GBS disease, and if baby appear well, should have a limited diagnostic evaluation. Infants of this gestational age should not be discharged before 48 hours at the earliest. See Figure 3 above.
Empirical Therapy for Infants with Positive Cerebrospinal Fluid (CSF)
There are no good prospective studies to indicate optimal choice of therapy in newborn infant with possible sepsis, but ampicillin and gentamycin are usually appropriate based on the usual susceptibilities of the predominant organisms causing early-onset sepsis (21). Infants with a positive CSF evaluation or with clinical signs of meningitis if the lumbar puncture (LP) has been deferred, should be treated with antibiotics which both penetrate the CSF are active against the likely organisms (See Table 2 below). If there is information from the maternal history suggesting an organism that is unlikely to respond to these antibiotics, empirical therapy should be adjusted appropriately. Blood cultures using modern automated systems are almost always positive by 48 hours (26). Therefore, if the laboratory results and clinical course do not indicate bacterial infection, therapy may be discontinued after 48 hours.
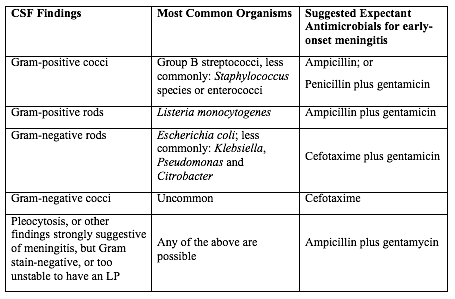
Table 2. Empirical therapy for infants with positive CSF.
Abbreviations: CSF (cerebrospinal fluid); LP (lumbar puncture);
Source: Canadian Paediatric Society, 2007
The majority of antibiotic courses are given to infants who eventually prove not to have has sepsis; strategies for further reduction of the duration of antibiotic therapy in such infants should be considered. For example, because gentamicin is usually now given once per day in the full-term infant, and ampicillin is given every 12 hours, the initial antibiotic order could be to give ampicillin for four doses every 12 hours and gentamicin for two doses every 24 hours, followed by reassessment after verification of culture results at 48 hours, and reordering the antibiotics in case of positive cultures or ongoing signs of sepsis (27).
GBS Vaccine Development
GBS is an important pathogen as it is the leading cause of neonatal deaths due to sepsis, meningitis or bacterial pneumonia. Although the development of an effective and safe GBS vaccine is on the agenda of many research labs, there is no GBS vaccine on the market yet. An effective vaccine is likely to prevent the majority of infant disease (both early and late onset), to avoid the limitations of intrapartum antibiotic prophylaxis and to be cost effective. A number of candidates, including capsular conjugate vaccines, have the potential to be successful vaccines. Phase II human studies with capsular conjugate vaccines have been completed successfully. Issues yet to be resolved include the safety and acceptability of vaccination during pregnancy, the durability of vaccine-derived immunity and regulatory issues required for licensure (28).
The World Health Organization (WHO) has identified the development of GBS vaccine for maternal immunization as a priority, based on the high unmet medical need, assessment of technical feasibility of vaccine development and the potential value of WHO involvement. In order to accelerate GBS vaccine development, the WHO has developed a vaccine development technology roadmap, to highlight priority activities for vaccine developers, researchers and funders. It has identified ‘preferred Product Characteristics’ which describe the vaccine characteristics that need to be considered in relation to the public health need (29).
The goal is to develop a vaccine for global use that can protect against GBS related stillbirth and invasive disease in neonates and young infants by immunizing pregnant women in the second and third trimester. The target is to provide 80% protection in fetuses/neonates against the combined risk of laboratory-confirmed GBS invasive disease-causing stillbirth and neonatal death. There are a number of challenges for GBS vaccine development. An effective vaccine needs to target over 90% of the current invasive disease isolates, either overcoming the diversity of GBS capsular types, or targeting protein expression polymorphism and prevalence. The potential exists for GBS invasive disease strain evolution and capsular switching, for which long-term strain composition is required.
The role of past GBS exposure and vaccines received in previous pregnancies needs to be determined. The vaccine’s immunogenicity on co-administration with other recommended vaccines in pregnancy and the impact on the immune responses to infants vaccines needs to be characterized, considering both the target antigen and similar carrier proteins. The impact of the vaccine in the presence of co-infections such as HIV and malaria in the pregnant women needs to be evaluated. An adjuvant, if used, should have a well-demonstrated safety profile in pregnant women.
There are currently no licensed vaccines that protect against GBS disease.
Neurodevelopment Impairment after Infant GBS Disease
GBS is a leading cause of infant meningitis, and almost one-fifth of GBS meningitis survivors experience moderate to severe neurodevelopment impairment (30). There is an additional, as yet unquantified burden associated with other invasive infant disease, such as GBS sepsis. It is critical to look toward improving the health and well-being of survivors of infant GBS disease, and supporting their families, for whom there are financial, social, psychological, and emotional impacts. See figure 4 below.
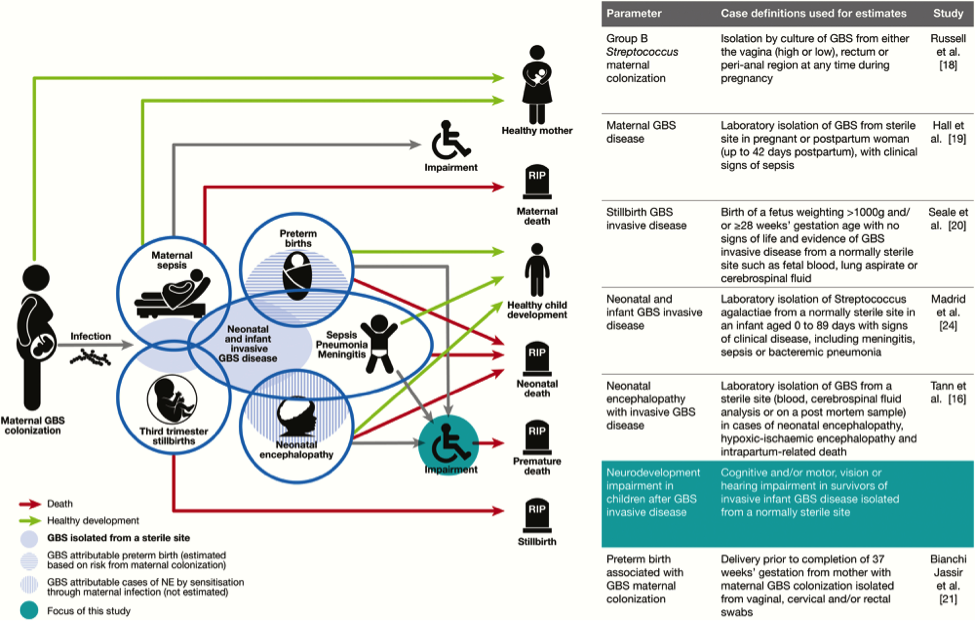
Figure 4. Neurodevelopmental impairment after infant group B streptococcal (GBS) disease in the disease schema for GBS. Abbreviations: GBS (group B streptococci); NE (neonatal encephalopathy).
Meningitis and sepsis can cause brain injury in term and preterm infants. Magnetic resonance imaging (MRI) findings consistently show cerebrovascular involvement, and abnormal findings on neonatal MRI have been clearly associated with poor neurodevelopment outcome at 2 years (30). This study found that all 9 cases of infant GBS meningitis in term babies had abnormal findings on MRI, of which 56% showed ischemic infarction (neonatal stroke). Furthermore, a case series of 8 term infants with GBS meningitis and ischemic stroke on MRI found 2 reports of neuroimaging in term infants with GBS meningitis have also found severe global cerebral vasculopathy and transverse myelitis (30). In preterm infants, inflammatory cytokines associated with infection increase the permeability of the blood-brain barrier and have an adverse effect on myelin-producing cells, resulting in periventricular leukomalacia, a condition strongly associated with neuro disability. In addition, sepsis causes brain injury indirectly through disseminated intravascular coagulopathy and hypotension; this is important to consider in cases of neonatal sepsis without meningitis. Furthermore, blood stream infection can have a sensitizing effect in the development of hypoxic-ischemic encephalopathy, which is also associated with neurodevelopmental impairment.
Rate of suspected neonatal sepsis after cervical ripening with a transcervical Foley catheter: Mechanical cervical ripening by transcervical Foley catheter is a common method of labor induction in cases of unfavorable cervix, and its efficacy and safety have been confirmed in multiple studies during the past decades (31). Nevertheless, it is unknown whether colonization with GBS affects the risk for maternal and neonatal infection in women undergoing cervical ripening with transcervical Foley catheter. This study reports the rates of maternal and neonatal infection morbidity after cervical ripening for labor induction with transcervical Foley catheter in women with GBS colonization (32). The rate of suspected neonatal sepsis was 5.4% slightly higher than described before in other works not related to GBS colonization, possibly due to a different definition for neonatal infection. This work suggests a generally favorable outcome with the use of transcervical Foley catheter in women with GBS colonization, although larger comparative studies are required to further improve counseling for this unique risk group.
Recommendations and Key Points
- For the purpose of neonatal management, the administration of intrapartum penicillin G, ampicillin, or cefazolin can provide adequate intrapartum antibiotic prophylaxis against neonatal early-onset GBS disease. For women at high risk of anaphylaxis to β-lactam antibiotics, clindamycin and vancomycin should be administered and will likely provide some protection against GBS early-onset-disease in exposed newborn infants. However, there is currently insufficient clinical efficacy evidence to consider the administration of these antibiotics equivalent to β-lactam antibiotics for the purpose of neonatal risk assessment.
- Risk assessment for early-onset GBS disease principles include the following (33):
- Separate consideration of infants born at >35 0/7 weeks’ gestation and those born at <34 6/7 weeks’ gestation;
- Infants born at >35 0/7 weeks’ gestation may be assessed for risk of early-onset GBS infection with a categorical algorithm by using multivariate models such as the Neonatal Early-Onset Sepsis Calculator or with enhanced clinical observation; and
- Infants born at <34 6/7 weeks’ gestation are at higher risk for early-onset infection from all causes, including group B streptococci, and may be best approached by using the circumstances to preterm birth to determine management.
- Early-onset GBS infection is diagnosed by blood or CSF culture. Common laboratory tests such as the CBC and C-reactive protein do not perform well in predicting early-onset infection, particularly among well-appearing infants at lowest baseline risk of infection.
- Evaluation for late-onset GBS disease should be based on clinical signs of illness in the infant. Diagnosis is based on the isolation of GBS from blood, CSF, or other normally sterile sites. Late-onset GBS disease occurs among infants born to mothers who had positive GBS screen results as well as those who had negative screen results during pregnancy. Adequate intrapartum antibiotic prophylaxis does not protect infants from late-onset GBS disease.
- Empirical antibiotic therapy for early-onset and late-onset GBS disease differs by postnatal age at the time of evaluation. Penicillin G is the preferred antibiotic for definitive treatment of GBS disease in infants; ampicillin is an acceptable alternative.
- The combination of ampicillin and gentamicin is the appropriate empirical antibiotic regimen for most infants who are at risk for early-onset sepsis. The empirical administration of additional broad-spectrum agents may be indicated in term infants who are critically ill until appropriate culture results are known.
- When blood cultures are sterile, antibiotic therapy should be discontinued by 36 to 48 hours of incubation unless there is clear evidence of site-specific infection.
Summary
GBS, a common commensal in the gut of humans and in the lower genital tract in women, remains an important cause of neonatal mortality and morbidity. The incidence of early onset disease has fallen markedly in countries that test women for carriage at 35 – 37 weeks of pregnancy and then offer intrapartum prophylaxis with penicillin during labor. Countries that do not test, but instead employ a risk factor approach, have not seen a similar fall. There are concerns about the effect on the neonatal microbiome of widespread use of antibiotic prophylaxis during labor, but so far the effects seem minor and temporary. Vaccination against GBS would be acceptable to most women and GBS vaccines are in the early stages of development.
While most babies recover from their GBS infection, some are stillborn, more die in the first weeks of life and others suffer lifelong disability. Despite efforts of many developed countries to prevent these infections, which are not preventable using current risk-based or screening strategies. Prevention strategies (intrapartum antibiotic prophylaxis) for early-onset invasive infant GBS disease are currently limited to developed countries, and only around the time of birth. Maternal GBS vaccination may be able to reduce the burden of GBS disease further, particularly GBS meningitis, which mainly presents as late-onset disease beyond 7 days of life.
References
- Puopolo KM, Madoff LC, Eichenwald EC. Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics 2005;115(5):1240-1246
- Van Dyke MK, Phares CR, Lynfield R, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med 2009;360(25):2626-2636
- Young BC, Dodge LE, Gupta M, Rhee JS, Hacker MR, Evaluation of a rapid, real-time intrapartum group B streptococcus assay. Am J Obstet Gynecol 2011;205(4):372.e1-e372
- Nanduri SA, Petit S, Smelser C, et al. Epidemiology of invasive early-onset and late-onset group B streptococcal disease in the United States, 2006 to 2015: multistate laboratory and population-based surveillance. JAMA Pediatr 2019;173(3):224-233
- American Academy of Pediatrics. Group B streptococcal infections. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. Itasca, IL: American Academy of Pediatrics; 2018:762-768
- Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis 2005 to 2014. Pediatrics 2016;138(6):e20162013pmid:27940705
- Kuzniewicz MW, Puopolo KM, Fischer A, et al. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr 2017;171(4):365-371 pmid:28241253
- Seale AC, Bianchi-Jassir F, Russell NJ, et al. Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis 2017;65(suppl_2):S200-S219 pmid:29117332
- Guilbert J, Levy C, Cohen R, Delacourt C, et al. Bacterial Meningitis Group. Late and ultra-late onset Streptococcus B meningitis: clinical and bacteriological data over 6 years in France. Acta Paediatr 2010;99(1):47-51 pmid:20002014
- Puopolo KM, Lynfield R, Cummings JJ. Management of infants at risk for group B streptococcal disease. Committee on Fetus and Newborn and Committee on Infectious Diseases. Pediatrics 2019;144(2):e20191881; DOI: https://doi.org/10.1542/peds.2019-1881
- Russell NJ, Seale AC, O’Driscoll M, et al. GBS Maternal Colonization Investigator Group. Maternal colonization with group B Streptococcus and serotype distribution worldwide: systematic review and meta-analyses. Clin Infect Dis 2017;65(suppl_2):S100-S111 pmid:29117327
- Baker CJ, Cary VJ, Rench MA, et al. Maternal antibody at delivery protects neonates from early onset group B streptococcal disease. J Infect Dis 2014;209(5): 781-788 pmid:24133184
- Patras KA, Nizet V. Group B streptococcal maternal colonization and neonatal disease: molecular mechanisms and prevention approaches. Front Pediatr 2018;6:27 pmid:29520354
- Escobar GJ, Puopolo KM, Wi S, et al. Stratification of risk of early-onset sepsis in newborns >34 weeks’ gestation. Pediatrics 2014;133(1):30-36 pmid:24366992
- Hornik CP, Benjamin DK, Becker KC, et al. Use of complete bold cell count in early-onset neonatal sepsis. Pediatr Infect Dis J 2012:31(8):799-802
- Mukhopadhyay S, Dukhovny D, Mao W, Eichenwald EC, Puopolo KM. 2010 perinatal GBS prevention guidelines and resource utilization. Pediatrics 2014;133(2):196-203
- Dhurasia MB, Mukhopadhyay S, Puopolo KM. Implementation of the sepsis risk calculator at an academic birth hospital. Hosp Pediatr 2018;8(5):243-250
- Joshi NS, Gupta A, Allan JM, et al. Management of chorioamnionitis-exposed infants in the newborn nursery using a clinical examination-based approach. Hosp Pediatr 2019;9(4):227-233
- Mukhopadhyay S, Puopolo KM, Clinical and microbiologic characteristics of early-onset sepsis among very low birth weight infants: opportunities for antibiotic stewardship. Pediatr Infect Dis J 2017;36(5):477-481
- Baltimore RS, Huie SM, Meek JI, Schuchat A, O’Brien KL. Early-onset neonatal sepsis in the era of group B streptococcal prevention. Pediatrics 2001;108(5):1094-1098
- Mtitimila EI, Cooke RW. Antibiotic regimens for suspected early neonatal sepsis. Cochrane Database Syst Rev 2004;Oct18:CD004495
- Metcalf BJ, Chochua S, Gertz RE Jr., et al. Active Bacterial Core Surveillance team. Short-read whole genome sequencing for determination of antimicrobial resistance mechanisms and capsular serotypes of current invasive Streptococcus agalactiase recovered in the USA. Clin Microbiol Infect 2017;23(8):574.e7-574.e14 pmid:28257899
- Moylett EH, Fernandez M, Rench MA, Hickman ME, Baker CJ. A 5-year review of recurrent group B streptococcal disease: lessons from twin infants. Clin Infect Dis 2000;30(2):282-287
- Matsubara K, Hoshina K, Kondo M, et al. Group B streptococcal disease in infants in the first year of life: a nationwide surveillance study in Japan, 2011-2015. Infection 2017;45(4):449-458
- Fernandez M, Rench MA, Albanyan EA, Edwards MS, Baker CJ. Failure of rifampin to eradicate group B streptococcal colonization in infants. Pediatr Infect Dis J 2001;20(4):371-376
- Kurlat I, Stoll BJ, McGowan JE Jr. Time to positivity for detection of bacteremia in neonates. J Clin Microbiol 1989;27(5):1068-1071
- Canadian Paediatric Society. Management of the infant at increased risk of sepsis. Paediatr Child Health 2007;12(10):893-898. Website: www.cps.ca
- Heath PT. An update on vaccination against group B streptococcus. Expert Rev Vaccines 2011;10(5):685-694
- Lin SM, Zhi Y, Ahn KB, Lim S, Seo HS. Status of group B streptococcal vaccine development. Clin Exp Vaccine Res 2018;7(1):76-81
- Kohli-Lynch M, Russel NJ, Seale AC, et al. Neurodevelopmental impairment in children after group B streptococcal disease worldwide: systematic review and meta-analyses. Clin Infect Dis 2017;65:S190-S199
- McMaster K, Sanchez-Ramos L, Kaunitz AM. Evaluation of a transcervical Foley catheter as a source of infection: a systematic review and meta-analysis. Obstet Gynecol 2015;126:539-551
- Ben-David A, Meyer R, Regev N, Mazaki-Tovi S. Maternal colonization with group B streptococcus and the risk for infection after cervical ripening with a transcervical Foley catheter. Obstet Gynecol 2021;137:662-663
- Puopolo KM, Benitz WE, Zaoutis TE. Committee on Fetus and Newborn; Committee on Infectious Diseases. Management of neonates born at >35 0/7 weeks’ gestation with suspected or proven early-onset bacterial sepsis. Pediatrics 2018;142(6):e201882894 pmid:30455342
Опубликован: 12 April 2021
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


