Overview of Blood Coagulation System
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
The concept of blood coagulation dates back to 1960's when Davie, Ratnoff and Macfarlane described the "waterfall" and "cascade" theories outlining the fundamental principle of cascade of proenzymes leading to activation of downstream enzymes (1). Haemostasis, defined as arrest of bleeding, comes from Greek, haeme (heme) meaning blood; and stasis meaning to stop. This thrombo-hemorrhagic balance is maintained in the body by complicated interactions between coagulation and fibrinolytic system as well as platelets and vessel wall. Coagulation is a dynamic process, and the understanding of the blood coagulation system has evolved over the recent years in anesthetic practice. Although the traditional classification of the coagulation provide more authentic description of the same.
The purpose of this document is to provide healthcare professionals the physiologic basis of hemostasis in order to and manage the abnormalities of the coagulation process and to interpret the diagnostic tests done for the same. Normal coagulation pathway represents a balance between the pro-coagulant pathway that is responsible for clot formation, and the mechanisms that inhibit the same beyond the injury site. Imbalance of the coagulation system may occur in the perioperative period or during critical illness, which may be secondary to numerous factors leading to a tendency of either thrombosis or bleeding.
Introduction
The coagulation process is usually under the inhibitory control of several inhibitors that limit the clot formation, thus avoiding the thrombus propagation. This delicate balance is interrupted whenever the pro-coagulant activity of the coagulaiton factors is increased, or the activity of naturally occuring inhibitors is decreased (2). Some of the thrombogenic and antithrombogenic component are listed below in Table 1.
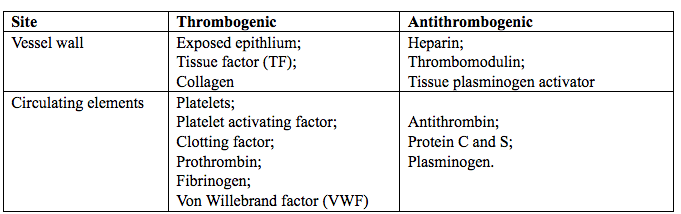
Table 1. Thrombogenic and antithrombogenic compoenents in the body.
It is important for a perioerative physician to understand the intricacies of two systems (more so in a preexisting hemotological disorder) that go side by side in maintaining the circulating blood in a fluidic state. Pathological situations requiring surgery or anesthesia or any other invasive procedure trigger the hemostatic system. This balance is also disturbed by trauma, cytokines or infectious agents. This the perioperiative period is at high risk for both pro-hemorrhagic and pro-thrombotic abnormalities. Hypoxia, hypothermia, metablolic acidosis and extracorporeal circulation may also further aggravate the situation (3).
Clotting Factors
Majority of clotting factors are precursors of proteolytic enzymes known as zymogens that ciruculate in an inactive form. The activation of each zymogen is depicted by suffixing letter "a" to the Roman numeral identifying that particular zymogen. Most of the procoagulants and anticoagulants are produced by liver except factor III, IV and VIII. These proteins undergo a post translational modification (vitamin K dependent γ carboxylation of glutamic acid residues) which enables them to bind calcium and other bivalent cations and participate in clottng cascade (4) see figure 1 below.
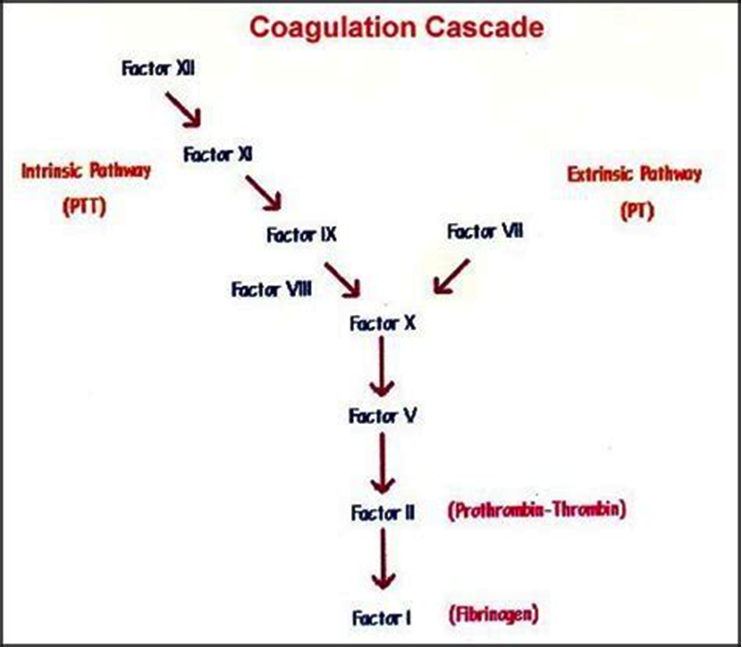
Figure 1. Coagulation cascade: an overview. Abreviated; PTT: Partial Thromboplastin Time; PT: Prothrombin Time.
Nomenclature of coagulation proteins is rather complex, see table 2 below. The first four of the 12 originally identified factors are referred to by their common names, i.e., fibrinogen, prothrombin, tissue factor (TF), and calcium are not assigned any Roman numerals. Factor VI no longer exists.
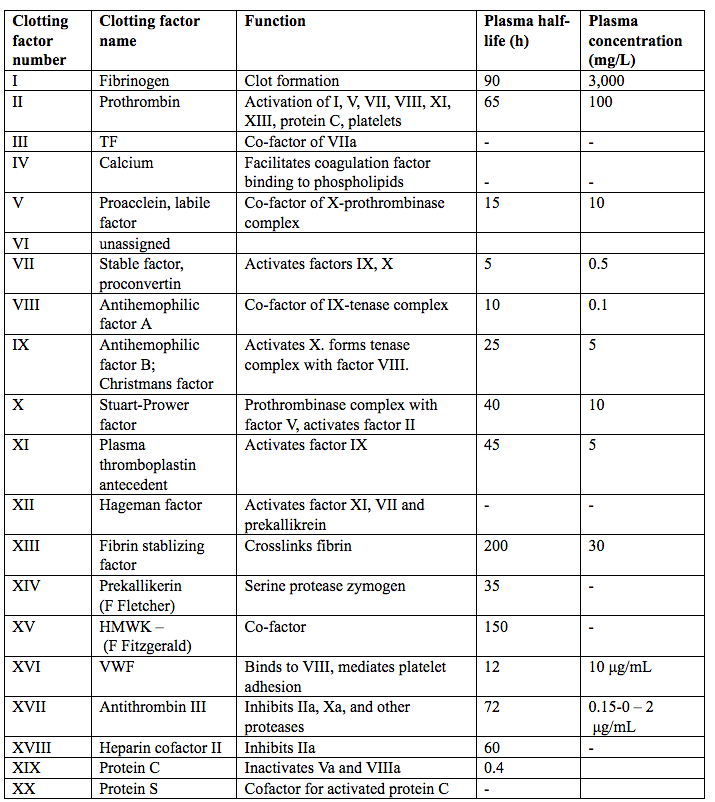
Table 2. Nomenclature of the coagulation proteins/clotting factors. Abbreviated; HMWK (high-molecular weight kininogen); VWF (Von Willebrand factor); TF (Tissue factor).
The more recently discovered clotting factors (e.g., prekallikerin and high-molecular-weight kininogen) have not been assigned Roman numerals. Some factors have more than one name. Factors V and VIII are also referred to as the labile factors because their coagulant activity is not durable in stored blood.
Clotting factors can also be classified into three groups (5): Fibrinogen family; vitamin K dependent proteins and contact family.

Table 3. Classification of coagulation factors. Abbreviated; HMWK - high-molecular-weight-kininogen.
Factor III and TF is a membrane bound procoagulant glycoprotein (MW47-kDa) present in the sub-endothelial tissue and fibroblasts and is not exposed to blood until disruption of the vessel wall (5). It is the primary initiator of coagulation in vivo. TF is localized predominantly to the tunica adventitia of blood vessels and a smaller quantity as circulating TF on monocytes. TF may be activated by physical injury (activation of Vessel Wall TF), by direct vascular injury or functional injury (activation of circulating TF), by hypoxia, sepsis, malignancy, inflammation (6).
Hypoxia up regulates the expression of P selection present in the α granules of platelets on the endothelium leading to recruitment of monocytes containing TF, thus initiating coagulation (7). With the exposure of TF to factor VII/VIIIa in the blood, it allows for the formation of TF-VIIIa complex and thus initiate the coagulation cascade.
Naturally Occurring Anticoagulants In The Body
The anticoagulants system exerts a regulatory role over the procoagulant activity in blood thus localizing the thrombus formation (8). The main anticoagulant mechanism naturally present in the body include the following: Antithrombin, Tissue factor plasminogen inhibitor, Protein C pathway and Protein Z dependent protease inhibitor/protein Z (PZI).
Antithrombin
Antithrombin (AT), previously known as AT III is the main inhibitor of thrombin. It is a serine protease inhibitor, which binds and inactivates thrombin, factor IXa, Xa, XIa and XIIa. The enzymatic activity of AT is enhanced in the presence of heparin. However, the plasma concentration of heparin is low and does not contribute significantly to the in vivo activation of AT. AT is activated by binding of heparin sulphate present on endothelial cell surface. AT binds coagulation factors in a ratio of 1:1 and this complex is removed by reticuloendothelial cells. Other thrombin inhibitor are heparin cofactor II, α 2 macroglobulin and α 1-antitrypsin (9).
Tissue Factor Plasminogen Inhibitor
It is a polypeptide produced by endothelial cells. It acts as a natural inhibitor of the extrinsic pathway by inhibiting TF-VIIa complex (10). Protein S enhances the interaction of factor Xa in the presence of calcium and phospholipids (11).
Protein C Pathway
The propagation phase of coagulation is inhibited by the Protein C pathway that primarily consist of four key elements:
- Protein C is a serine protease with potent anticoagulant, profibrinolytic and anti-inflammatory properties. It is activated by thrombin to form activated protein C (APC) and acts by inhibiting activated factor V and VIII (with Protein S and phospholipids acting as cofactors).
- Thrombomodulin - A transmembrane receptor on the endothelial cells, it prevents the formation of the clot in the undamaged endothelium by binding to the thrombin.
- Endothelial protein C receptor is another transmembrane receptor that helps in the activation of Protein C.
- Protein S is a vitamin K-dependent glycoprotein, synthesized by endothelial cells and hepatocytes. It exists in plasma as both free (40%) and bound (60%) forms (bound to C4b-binding protein). The anticoagulant activity is by virtue of free form while the bound form as an inhibitor of the complement system and is up regulated in the inflammatory states, which reduce the Protein S levels thus resulting in procoagulant state. It functions as a cofactor to APC in the inactivation of FVa and FVIIIa. It also causes direct reversible inhibitor of the prothrombinase (FVa - FXa) complex (12).
Protein Z dependent protease inhibitor/protein Z (PZI)
It is a recently described component of the anticoagulant system that is produced in the liver. It inhibits factor Xa in reaction requiring PZ and calcium (13).
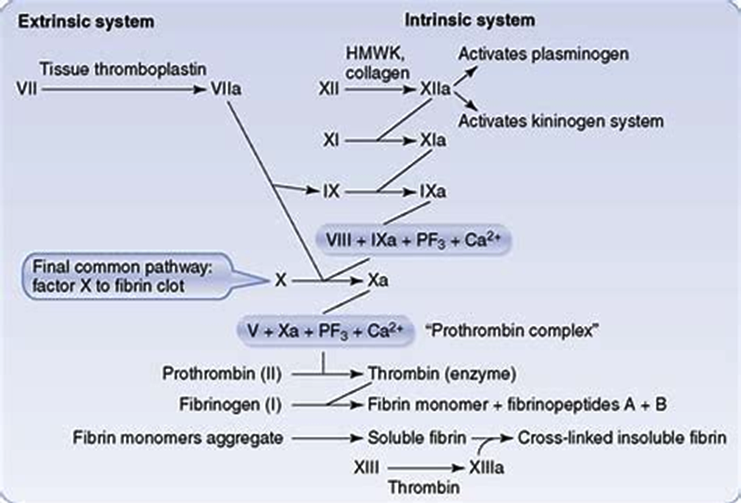
Figure 2. Extrinsic and Intrinsic pathways. Both converging on prothrombin complex and thrombin
Coagulation Pathways
It has been traditionally classified into intrinsic and extrinsic pathways, both of which converge on factor X activation. The classical theory of blood coagulation is particularly useful for understanding the In vitro coagulation tests, but fails to incorporate the central role of cell-based surfaces in In vivo coagulation process (14). Increasingly contact activation for in vivo hemostasis does not get support from following observations. Persons lacking FXII, prekallikrein, or high-molecular-weight kininogen do not bleed abnormally. Second, patients with only trace quantities of FXI can withstand major trauma without unusual bleeding, and those who completely lack factor XI (hemophilia C) exhibit mild hemorrhagic disorder. Deficiencies of FVIII and FIX (both intrinsic pathway factors) lead to hemophilia A and B, respectively, however the classic description of two pathways of coagulation leave it unclear as to why either type of hemophiliac (A or B) cannot simply clot blood via the unaffected pathway.
To answer all this, the modern time-based structuring of blood coagulation provides more authentic description of the coagulation process. It is now appreciated that the classic theories may provide only a reasonable model of In vitro coagulation tests (i.e., aPTT and PT).
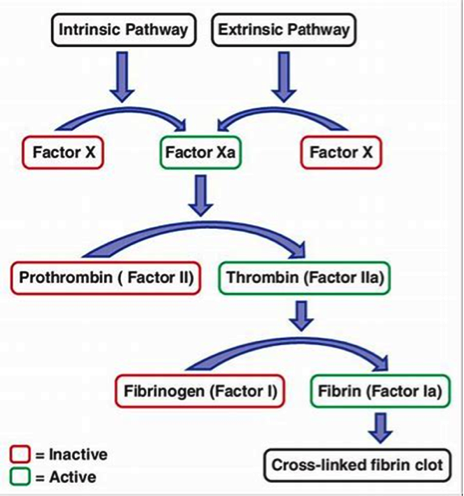
Figure 3. Intrinsic and extrinsic pathways. Both converging on factor X activation
Three Coagulation pathways
Extrinsic pathway
It is considered as the first step in plasma mediated hemostasis. It is activated by TF, which is expressed in the subendothelial tissue (15). Under normal physiological conditions, normal vascular endothelium minimizes contact between TF and plasma procoagulants, but vascular insult expose TF which binds with factor VIIa and calcium to promote the conversion of factor X to Xa (16).
Intrinsic pathway
It is a parallel pathway for thrombin activation by factor XII. It begins with factor XII, HMW kininogen, prekallikrein and factor XI, which results in activation of factor XI. Activated factor XI further activates factor IX, which then acts with its cofactor (factor VIII) to form tenase complex on a phospholipid surface to activate factor X (17).
Common pathway
Activated factor X along with its cofactor (factor V), tissue phospholipids, platelet phospholipids and calcium forms the prothrombinase complex which converts prothrombin to thrombin. This thrombin further cleaves circulating fibrinogen to insoluble fibrin and activates factor XIII, which covalently crosslinks fibrin polymers incorporated in the platelet plug. This creates a fibrin network which stabilizes the clot and forms a definitive secondary hemostatic plug (18).
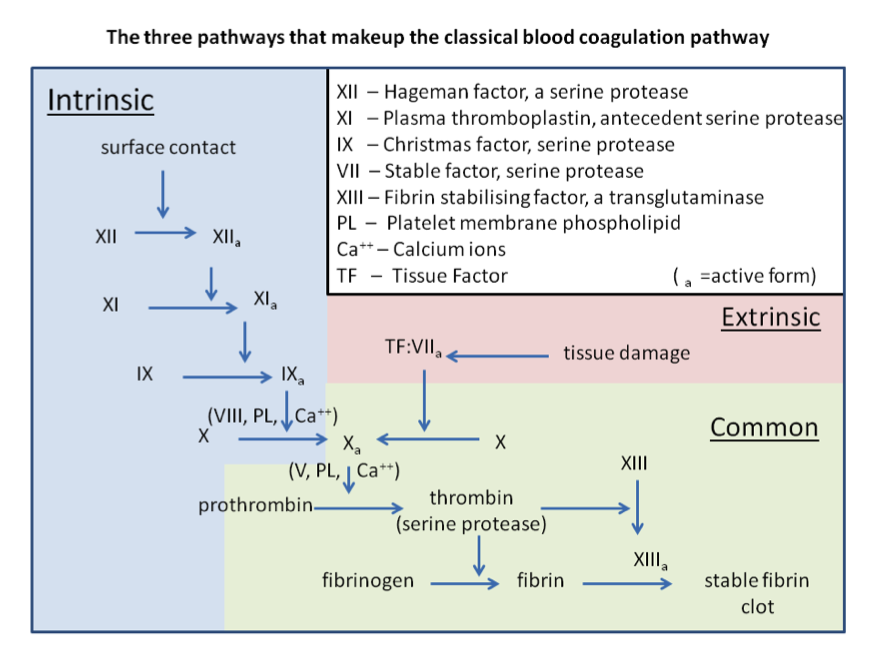
Figure 4. Three pathways of normal coagulation.
Current Concept of Coagulation
Current evidence supports the understanding that intrinsic pathway is not a parallel pathway but indeed it augments thrombin generation primarily initiated by the extrinsic pathway (19).
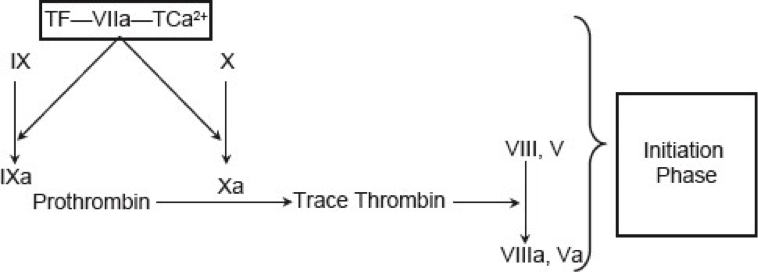
Figure 5. Current concept of coagulation (initiation phase).
Initiation: It occurs by expression of TF in damaged vessel which binds factor VIIa to activate factor IX and factor X. This activation of factor IX by TF-VIIa complex servers as the bridge between classical extrinsic and intrinsic pathways. Factor Xa then binds to factor II to form thrombin (factor IIa). Thrombin generation through this reaction is not robust and can be effectively terminated by TF pathway inhibitor (see figure 5 above).
Amplification: Since the amount of prothrombin generated is not sufficient, therefore numerous positive feedback loops are present that bind thrombin with platelets. Thrombin that is generated in the initiation phase further activates factor V and factor VIII, which serves as a cofactor in prothrombinase complex and accelerates the activation of Factor II by F Xa and of F Xa by F IXa, respectively (21).
Propagation: The accumulated enzyme complexes (tenase complex and prothrombinase complex on platelet surface support robust amounts of thrombin generation and platelet activation.
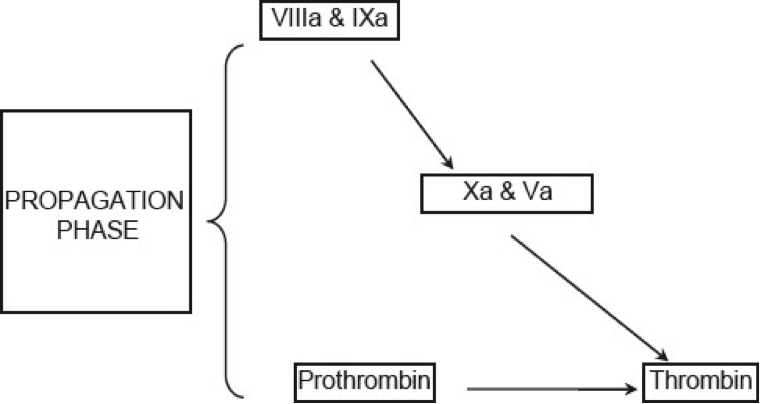
Figure 6. Current concepts of coagulation (Propagation phase).
This ensures continuous generation of thrombin and subsequently fibrin to form a sufficiently large clot (See figure 6 above).
Stabilization: Thrombin generation leads to activation of factor XIII (fibrin stabilizing factor) which covalently links fibrin polymers and provides strength and stability to fibrin incorporated in platelet plug. In addition, thrombin activates thrombin activatable fibrinolysis inhibitor (TAFI) that protects the clot from fibrinolysis (20).
Standardization of Prothrombin Time (PT)/International Normalized Ratio (PT/INR)
The prothrombin time (PT) represents the most commonly used coagulation test in clinical laboratories. The PT is mathematically converted to the international normalized ratio (INR) for use in monitoring anticoagulant therapy with vitamin K antagonists such as warfarin in order to provide test results that are adjusted for thromboplastin and instrument used. The INR is created using two major PT 'correction factors', namely the mean normal PT (MNPT) and the international sensitivity index (ISI).
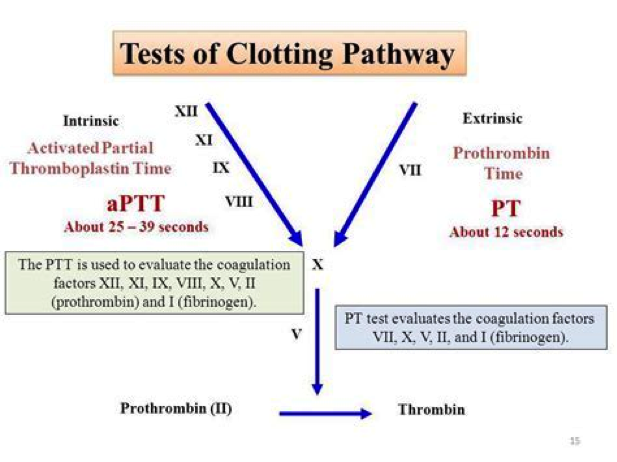
Figure 7. Tests of clotting pathways. Abbreviated aPTT: Activated Partial Thromboplastin Time; PT: Prothrombin Time.
Manufacturers of regents and coagulometers have made some efforts to harmonizing INRs, for example, by tailoring reagents to specific coagulometers and provide associated ISI values. Thus, two types of ISIs may be generated, with one being a 'general' or 'generic' ISI and others being reagent/coagulometer-specific ISI values. Although these play a crucial role in improving INR results between laboratories, these laboratories reported INR values are known to still differ, even when laboratories use the same thromboplastin reagent and coagulometer. Moreover, ISI values for a specific thromboplastin can vary among different models of coagulometers from a manufacturer using the same method for clot identification (22).
All these factors can be sources of error for INR reporting, which in turn can significantly affect the patient management. A prolonged PT might be due to one or more coagulation factor defects in the TF or common pathways, or might indicate the presence of inhibitors against these factors (23). In order to differentiate between coagulation factor deficiency and the presence of inhibitors, a mixing study is performed. If the PT is corrected after the addition of normal plasma, congenital, or acquired coagulation factor(s) deficiency is implied, whereas, if the PT remains prolonged after the addition of normal plasma raises the possibility of inhibitors (24).
Acquired defects of coagulation factors prolonging the PT are commonly observed in people with vitamin K deficiency (25). Alternatively, vitamin K antagonists (VKAs) such as warfarin, as prescribed to prevent thromboembolic events, hamper gamma-carboxylation of vitamin K-dependent coagulation factors (VKDCFs) and thus reduce their activity and also prolong PT (26). However, in the case of VKAs, prolongation of the PT into a therapeutic range is desired. Given that the synthesis of most coagulation factors is restricted to the liver, disorders of this organ can be associated with a decrease of most coagulation factors, resulting in prolonged PT and/or APTT. Prolonged PT may also occur in patients with acquired FX deficiency due to amyloidosis, especially amyloid light-chain (AL) amyloidosis (27).
The attempts to better standardize results of PT testing began in 1962, leading to the introduction of British Comparative Thromboplastin (BCT) as the reference thromboplastin in 1969. Following the standardization process, the World Health Organization (WHO) introduced the International Normalized Ratio System (INR) in 1983 (28). This standardization system provides a simple way to interpret PT results independently of the thromboplastin used.
International Normalized Ratio (INR)
Initial efforts to standardize PT test results by reporting PT results as PT ratios were unable to completely resolve the issue of variability. The PT ratio, which is the patient's PT over a mean normal PT ratio, did not fully eliminate discrepancies between results: the test was still affected by variations in thromboplastin and could not be used to harmonize results for patients receiving vitamin K antagonists. The INR instead reflects a mathematical calculation using a PT ratio as further adjusted with a correction factor called the international sensitivity index (ISI) (29).
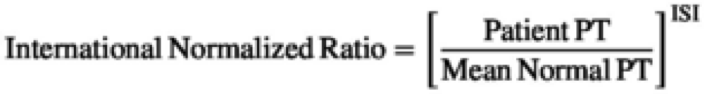
Figure 8. Mathematical calculations using a PT ratio reflection International Normalized Ratio. Abbreviated; PT: prothrombin time; ISI: international sensitivity index.
According to WHO standards, an acceptable ISI value for thromboplastin reagents for manual methods is between 0.9 and 1.7 (30). However, due to a more severe decrease in FVII levels than other vitamin K-dependent coagulation factors, INR is not an appropriate index to evaluate the patient's condition at the onset of anticoagulation with vitamin K antagonists.
Ordering Coagulation Profile
Commonly used tests for hemostasis are as follows:
- Platelet count - It gives an account of the number of platelets in the volume of blood (normal value: 150 to 400 X109/L). Low platelet count occurs in thrombocytopenia. It could be primary (isolated thrombocytopenia) without any other underlying cause, or it could be secondary with associated conditions such as HIV (human immunosuppressive virus), HCV (hepatitis C virus), SLE (systematic lupus erythematosus), CLL (chronic lymphocytic leukemia). Certain drugs cause drug-induced thrombocytopenia, e.g., aspirin, ethanol, and NSAIDs (non-steroidal anti-inflammatory drugs).
- Prothrombin Time (PT) - It is also reported along with laboratory control (normal range: 11 to 24s). It measures the extrinsic pathway (factor VII) and common pathway. It is prolonged in vitamin K deficiency, vitamin K antagonist therapy (warfarin), and factor VII deficiency.
- International Normalized Ratio (INR) - Normal range is 0.9 to 1.2. It is used to monitor warfarin therapy and for the assessment of hepatic function.
- Activated Partial Thromboplastin Time (aPTT) - It is reported against a normal control (normal range: 22 to 35s). It measures the activity of the common pathway and intrinsic pathway (factors VIII, IX, XI, XII). It is also used to monitor heparin therapy. It is prolonged in hemophilia A (factor VIII deficiency) and B (factor IX deficiency).
- Other Tests - Fibrinogen, D-Dimers, Specific factor assays (factor VIII), Lupus anticoagulant, Tests for thrombophilias (activated protein C resistance) and von Willebrand tests (VWF antigen, Ristocetin cofactor activity, factor VIII).
In a patient suspected of coagulation disorder, the investigations should be pre-planned. History and physical examination findings are significant and help decide whether the patient has a vascular defect, platelet defect, or a defect in one of the coagulation factors. It also gives clues of the hereditary or acquired nature of the disorder and narrows down the tests performed. The preliminary tests ordered include platelet count, PT and aPTT. Further course of action is decided based on the results of these tests.
Summary
Haemostasis is a complex physiological process, maintaining the fluidity of blood and is regulated by delicate balance existing between thrombogenic and anti-thrombogenic mechanisms present in the body. Imbalance between the two components predisposes a patient to either bleed or present with thrombosis. The physiology of the same therefore, needs to be understood to predict the pathological and clinical consequences of the same before implementing any pharmacological interventions. Accurate reporting of PT/INR results has a direct effect on the management of patients undergoing vitamin K antagonists therapy. An appropriate standardization process, can significantly improve the accuracy of reported results. Verification and if necessary, calibration of reagent/coagulometer-specific, and generic ISIs, prior to clinical use is mandatory, and play a crucial role in improving PT/INR results.
Suggested Reading
- Vitamin K Deficiency Bleeding
http://www.womenshealthsection.com/content/obsnc/obsnc014.php3 - Managing von Willebrand Disease in Women
http://www.womenshealthsection.com/content/gyn/gyn036.php3
References
- Achneck HE, Sileshi B, Parikh H, Milano CA, et al. Pathophysiology of bleeding and clotting in the cardiac surgery patient: from vascular endothelium to circulatory assist device surface. Circulation 2010;122(20):2068-2077
- Previtali E, Bucciarelli P, Passamonti SM, Martinelli I. Risk factors for venous and arterial thrombosis. Blood Transfus 2011;9(2):120-138
- Bombeli T, Spahn DR. Updates in perioperative coagulation: Physiology and management of thromboembolism and haemorrhage. Br J Anaesth 2004;93:275-287
- Monroe DM 3rd, Hoffman M, Roberts HR. Molecular biology and biochemistry of the coagulation factors and pathways of hemostasis; Williams Hematology. 8th edition; 2010: pp.614-616; New York,, NY: McGraw-Hill Professional Publishing.
- Mackman N, Tilley RE, Key NS. Role of extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol 2007;27(8):1687-1693
- Manly DA, Boles J, Mackman N. Role of tissue factor in venous thrombosis. Annu Rev Physiol 2011;73:515-525
- Myers DD, Hawley AE, Farris DM, Wrobleski SK, et al. P-selection and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg 2003;38(5):1075-1089
- Covin BT. Physiology of haemostasis. Vox Sang 2004;87(Suppl 1):43-46
- Ejiofor JA. Anticlotting mechanism I: Physiology and pathology. Contin Educ Anesth Crit Care Pain 2013;13:87-92
- Price GC, Thompson SA, Kam PC. Tissue factor and tissue factor pathway inhibitor. Anaesthesia 2004;59:483-492
- Dham AE, Sandset PM, Rosendaal R. The association between protein S levels and anticoagulant activity of tissue factor pathway inhibitor type 1. J Thromb Haemost 2008;6:393-395
- Rigby AC, Grant MA. Protein S: a conduit between anticoagulation and inflammation. Crit Care Med 2004'32(5 Suppl):S336-341
- Corral J, Gonzalez-Conejero R, Hernandez-Epinosa D, Vicente V. Protein Z/Z-dependent protease inhibitor (PZ/ZPI) anticoagulant system and thrombosis. Br. J Haemstol 2007;137(2):99-108
- Bombeli T, Spahn DR. Updates in perioperative coagulation: physiology and management of thromboembolism and haemorrhage. Br. J Anaeth 2004;93(2):275-287
- Lasne D, Jude B, Susen S. From normal to pathological hemostasis. Can J Anaesth 2006;53(6 Suppl): S2-11
- Owens AP III, Mackman N. Tissue factor and thrombosis: The clot starts here. Thromb Haemost 2010;104(3):432-439
- Hall JE. Guyton and Hall Textbook of Medical Physiology: Enhanced E-Book. 11th ed. Philadelphia: Elsevier Health Sciences; 2010. Hemostasis and blood coagulation; pp.457-459
- Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 8th ed. Philadelphia, PA: Saunders Elsevier; 2010. Hemodynamic disorders, thromboembolic disease and shock; pp. 118-120
- Triplett DA. Coagulation and bleeding disorders: review and update. Clin Chem 2000;46(8 Pt 2):1260-1269
- Lasne D, Jude B, Susen S. From normal to pathological hemostasis. Can J Anaesth 2006;53(6 Suppl):S2-11
- Palta S, Sarora R, Palta A. Overview of the coagulation system. Indian J Anaesth 2014;58(5):515-523
- Dorgalaleh A, Favaloro EJ, Bahrani M, Rad F. Standardization of prothrombin time/ International Normalized Ratio (PT/INR). Int. J. Lab. Hematol 2021;43:21-28
- Dorgalaleh A, Daneshi M, Rashidpanah J, Yasaghi ER. An overview of Hemostasis. Congenital Bleeding Disorders. Diagnosis and Management. Cham, Switzerland: Springer; 2018;3-26
- Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc 2007;82(7):864-873
- Rad F, Hamidopour M, Dorgalaleh A, et al. The effect of demographic factors and VKORC1 1639 G>A genotypes on estimated warfarin maintenance dose in Iranian patients under warfarin therapy. Indian J Hemtol Blood Transfus 2019;35:167-171
- De Caterina R, Husted S, Wallentin L, et al. Vitamin K antagonists in heart disease: current status and perspectives (Section III). Thromb Haemost 2013;110 (12):1087-1107
- Choufani EB, Sanchorawala V, Ernst T, et al. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood 2001; 97(6):1885-18887
- Johnson SA, Vazquez SR, Fleming R, Lanspa MJ. Correction factor to improve agreement between point-of-care and laboratory International Normalized Ratio values. Am J Health Syst Pharm 2017;74 (1):e24-e31.
- Meijer P, Kynde K, van den Besselaar AM, Van Blerk M, Woods TA. International normalized ratio (INR) testing in Europe: between-laboratory comparability of test results obtained by Quick and Owren reagents. Clin Chem Lab Med 2018;56(10):1698-1703
- Pollack CV. Introduction to direct oral anticoagulants and rationale for specific reversal agents. Am J Emerg Med 2016;34(11):1-2
Published: 28 December 2021
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


