Neonatal Abstinence Syndrome
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Neonatal Abstinence Syndrome (NAS) is a result of the sudden discontinuation of fetal exposure to substances that were used for or abused by the mother during pregnancy. Withdrawal from licit or illicit substances is becoming more common among neonates in both developed and developing countries. Substance abuse among pregnant woman is a major public health issue. Both prescription opioid use and illicit opioid abuse have increased dramatically in recent years. Prolonged in utero drug exposure may result in NAS, an acute multisystemic clinical entity that occurs in the first days of life. NAS is a growing problem in the United States. NAS occurs when newborn babies experience withdrawal after being exposed to drugs in the womb. Fortunately, NAS is preventable if an expectant mother receives proper care and treatment.
The purpose of this document to review available evidence for Neonatal Abstinence Syndrome (NAS) assessment tools, non-pharmacological interventions, and pharmacologic management of opioid exposed infants. Opioid medications such as morphine or methadone are recommended as first-line therapy, with phenobarbital or clonidine as second-line adjunctive therapy. Further research is needed to determine best practice for assessment, non-pharmacologic intervention, and pharmacologic management of infants with NAS in order to improve outcomes. The lessons learned from prenatal alcohol exposure might be relevant for opioids. Full consideration must be given to the to the postnatal environment of children with prenatal opioid exposures, which might include social and economic complexities that adversely impact child development.
Incidence
The rates of Neonatal Abstinence Syndrome (NAS) increased 5 times between year 2000 and the year 2013, in the United States of America (USA). As of 2014, there was an average of one infant born with NAS every 15 minutes in the USA, totaling about 32,000 infants, accounting for an estimated $1.5 billion in healthcare spending that year alone (1). Among six identified states that mandated NAS reporting laws during 2013 – 2017, NAS incidence could be quantified to inform programs and services. However, differences in reporting methods and case definitions might influence states' abilities to monitor NAS incidence.
From 2004 – 2014, the incidence of NAS in the USA increased 433%, from 1.5 to 8.0 per 1,000 hospital births (2). The US opioid crisis is the public health emergency of our time and requires urgent public health action to monitor and protect the most vulnerable Americans. We have witnessed a startling death toll in 2017 with 70,237 drug overdose in the US, of which two-thirds involved were opioids (3). The devastating consequences of this epidemic for mothers and infants have received less attention. Increases in opioid use and misuse in pregnancy have propelled the increases in the general population; at the delivery hospitalization, there were 4 times as many women with an opioid use disorder in 2014 compared with year 1999 (3).
Background
NAS is caused by abrupt discontinuation of fetal exposure to licit or illicit drugs chronically consumed by the mother during pregnancy and transmitted to the fetus through placenta. It usually requires prolonged hospitalization and may have long-term effects. The interplay of many factors contributes to its clinical heterogeneity, and its pathophysiology has not been fully unveiled. One of the most effective prevention strategies is to improve preconception health care, and to educate both patients and providers about appropriate use of prescription drugs during pregnancy. Though there have been some recent initiatives to reduce rates of opioid use, few have included a focus on pregnant women and their babies. Screening of pregnant women can also be an effective prevention strategy by determining who may need additional care or treatment for opioid use.
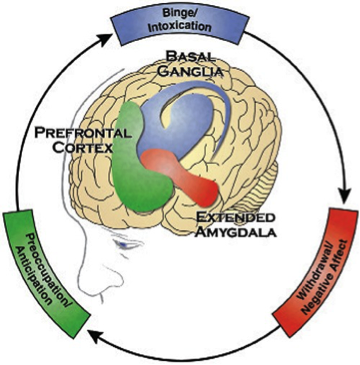
Figure 1. The three stages of the Addiction Cycle and the Brain Regions associated with them.
Identifying Babies Born Exposed to Opioids
Healthcare providers should closely watch pregnant women with opioid use to carefully manage the medical care for both mother and baby during pregnancy and after delivery. One of the concerns is withdrawal symptoms in the newborn. This study found that identifying babies exposed to opioids and gabapentin in the womb may result in better treatment and shorter hospital stays (3). Opioids and gabapentin are medicines used to treat some types of pain and to treat opioid use disorder. Main findings are:
- Laboratory screening of all women after delivery allowed healthcare providers to identify more newborns with withdrawal symptoms.
- Identifying exposure as soon as possible allowed for better treatment and likely resulted in shorter hospital stay for newborns.
- Before laboratory screening of all women, newborns with co-exposure began treatment around day 20. After screening began, newborns began treatment around day 14 of life.
- Before laboratory screening of all women, newborns with co-exposure has an average hospital stay of 58 days. After screening began, newborns has an average hospital stay of 48 days.
- Figuring out which substances newborns are exposed to during pregnancy can help decide their care and treatment. Laboratory screening of all women after delivery can be considered as a complement to continued discussion about medicine and substance use between healthcare providers and patients.
Clinical Presentation of Opioid Withdrawal
The clinical presentation of NAS with the opioid, the maternal drug history (including timing of the most recent use of drug before delivery), maternal metabolism, net transfer of drug across the placenta, placental metabolism, infant metabolism and excretion, and other factors (5). In addition, maternal use of other drugs and substances such as cocaine, barbiturates, hypnotics-sedatives, and cigarettes may influence the severity and duration of NAS. Because opioid receptors are concentrated in the central nervous system (CNS) and the gastrointestinal tract, the predominant signs and symptoms of pure opioid withdrawal reflect CNS irritability, autonomic overactivity, and gastrointestinal tract dysfunction. Excess environmental stimuli and hunger will exacerbate the perceived severity of NAS.
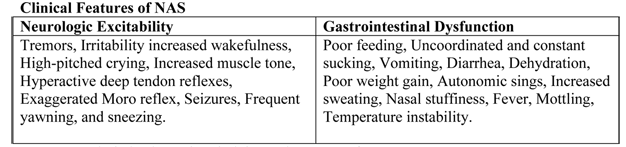
Table 1. Neurological and gastrointestinal signs and symptoms of NAS.
Onset of signs attributable to neonatal withdrawal from heroin often begins within 24 hours of birth, whereas withdrawal from methadone usually commences around 24 to 72 hours of age (6). For both opioids, evidence of withdrawal may be delayed until 5 to 7 days of age or later, which is typically after hospital discharge. For infants exposed to buprenorphine, the study found that onset of withdrawal peaked at 40 hours and that signs were most severe at 70 hours of age (6). The different time courses reflect variations in the half-lives of drug elimination. However, if one week or longer has elapsed between the last maternal opioid use and delivery of the infant, the incidence of neonatal withdrawal is relatively low (7). The incidence and severity of NAS are greater in infants exposed to methadone compared with those exposed to buprenorphine or heroin. Still, severe withdrawal has been described in 0 to 50% of buprenorphine-exposed infants (8). In the acute phase, seizures have occurred in 2% to 11% of infants withdrawing from opioids, however, abnormal EEG results without overt seizure activity have been reported in >30% of neonates. Subacute signs of opioid withdrawal may last up to 6 months (9).
Seizures also may be associated with withdrawal from a variety of non-narcotic drugs (e.g., barbiturates, alcohol, and sedative-hypnotics (10). The mechanism and significance of seizures associated with withdrawal are unclear. Withdrawal from ethanol begins early, in general, during the first the first 3 to 12 hours after delivery. Diagnosis of sedative withdrawal is more difficult, because classically it appears after the few days of life. Barbiturate withdrawal has a median onset of 4 to 7 days, but a wide range from days 1 through 14. Other sedative-hypnotics have exhibited even later onset, including as late as day 12 for diazepam and day 21 for chlordiazepoxide.
Studies of the relationship between maternal methadone dose and the incidence and severity of NAS have provided contradictory findings. Some studies demonstrated that larger maternal methadone dosages in late pregnancy were associated with greater neonatal concentrations and increased risk of withdrawal (11), but others refuted a correlation (10). This lack of consensus is explained in part by different approaches to the management of antenatal methadone maintenance therapy. There were substantial variations in the mean and range of daily methadone dose in the populations studied. Studies that found no correlation tended to enroll infants born to mothers who had been prescribed higher doses of methadone (50 – 200 mg/day), whereas those that did note a relationship between maternal dose and NAS sequelae reported lower maternal doses (e.g. <50 mg/day) or included women undergoing partial detoxification (12). Another potential explanatory factor is the significant interindividual variability in maternal methadone metabolism (13). As a result, cumulative fetal exposure can be expected to vary among infants born to mothers on equivalent methadone regimens.
Methadone concentrations in cord blood and at 48 hours of age, as well as the rate of decline in neonatal serum concentration, appear to correlate with NAS signs (14). This study found that infants who required rescue treatment had lower cord blood methadone concentrations and that, in all but 1 infant, methadone concentrations were undetectable in the serum at 48 hours (14). It is noted that faster declines in postnatal blood methadone concentrations were associated with more severe CNS withdrawal.
Preterm Infants
Preterm infants have been described as being at lower risk of drug withdrawal with less severe and/or prolonged courses. Infants born at <35 weeks' gestation whose mothers received methadone maintenance had significant lower total and CNS abstinence scores than did term infants of mothers receiving similar methadone doses (15). Lower gestational age correlated with a lower risk of neonatal withdrawal. The apparent decreased severity of sings in preterm infants may be related to developmental immaturity of the CNS, differences in total drug exposure, or lower fat depots of drug. Alternatively, the clinical evaluation of the severity of abstinence may be more difficult in preterm infants because scoring tools to describe withdrawal were largely developed in term or later preterm infants (16).
Abuse of Multiple Drugs
The abuse of multiple drugs during pregnancy is not uncommon, but its effect on the occurrence and severity of neonatal abstinence is controversial (17). Intrauterine exposure to certain drugs may cause congenital anomalies and/or fetal growth restriction, increase risk of preterm birth, produce signs of withdrawal or toxicity in the neonate, or impair neuro-development (18).
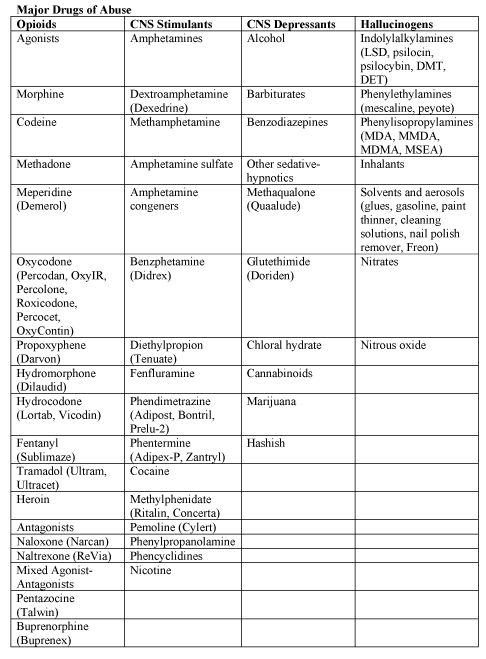
Table 2. Abbreviations: DET (dimethyltryptamine); DMT (dimethyltryptamine); LSD (lysergic acid diethylamide); MDA (methylenedioxyamphetamine); MDEA (3,4-methlenedioxyethamphetamine), MDMA (3,4-methylenedioxymethaphtamine [ecstasy]); and MMDA (3-methoxy-4, 5-methlenedioxyamphetamine.
Fetal exposure to marijuana, the illicit drug most commonly used by pregnant women, does not cause clinically important neonatal withdrawal signs but may have subtle effects on long-term neurobehavioral outcomes (19).
Differential Diagnosis
The presence of maternal characteristics known to be associated with drug abuse during pregnancy can be considered an indication to screen for intrauterine drug exposure. These characteristics include absent, late, or inadequate prenatal care; a previously documented or admitted history of drug abuse; a previous unexplained late fetal demise; precipitous labor; abruptio placentae; hypertensive episodes; severe mood swings; cerebrovascular accidents; myocardial infarction; and repeated spontaneous abortions (20). The legal implications of testing and the need for consent from the mother may vary among the states. Each hospital should consider adopting a policy for maternal and newborn screening to avoid discriminatory practices and to comply with local laws.
Withdrawal signs in the newborn may mimic other conditions, such as infection, hypoglycemia, hypocalcemia, hyperthyroidism, intracranial hemorrhage, hypoxic-ischemic encephalopathy, and hyperviscocity (21). If none of these diagnoses is readily apparent, a detailed maternal drug history should be obtained that includes interviewing the mother about drug use and abuse by her partner, friends, and parents, in addition to queries about the mother's prescription and non-prescription drug use. Screening is most commonly accomplished by using neonatal urine specimens. A urine sample must be collected as soon as possible after birth, because many drugs are rapidly metabolized and eliminated. Even so, a positive urine screening result may only reflect recent drug use. Alcohol is detectable in neonatal urine for 6 to 16 hours after the last maternal ingestion. Amphetamines, benzodiazepines, cocaine metabolites, and opioids are usually cleared withing 1 to 3 days after birth. Marijuana and cocaine metabolites may be detectable for weeks, depending on maternal usage.
Drugs that are excreted in the hepatobiliary system as well as drugs excreted by the fetal kidneys into the amniotic fluid are concentrated in meconium. Hence, meconium analysis is most useful when the history and clinical presentation strongly suggest neonatal withdrawal, but the maternal and neonatal urine screening results are negative. Drawbacks of testing for drugs in meconium are that it is not typically performed by hospitals and that results are often not available for days to weeks. Meconium must be collected before it is contaminated by transitional, human milk, or formula stools – otherwise, the assay may not be valid, or the reference laboratory may reject the sample. Assay of meconium, although not conclusive if the results are negative, is more likely to identify infants of drug-abusing mothers than is the testing of infant or maternal urine. Other specimens that have been tested in research laboratories are maternal and neonatal hair (22).
Recently, testing of umbilical cord tissue by using drug class-specific immunoassays was shown to be in concordance with testing of paired meconium specimens at the rates of 97%, 95%, 99% and 91% for detection of amphetamines, opiates cocaine, and cannabinoids, respectively (23). The availability of this tissue from the moment of birth (in contrast to inherent delay in collecting urine or meconium) may foster the adoption of this method of testing.
Assessment and Non-Pharmacologic Treatment
The modified Neonatal Abstinence Scoring System (Table 3), is the predominant tool used in the United States. This more comprehensive instrument assigns a cumulative score based on the interval observation of 21 items relating to signs of neonatal withdrawal (24).
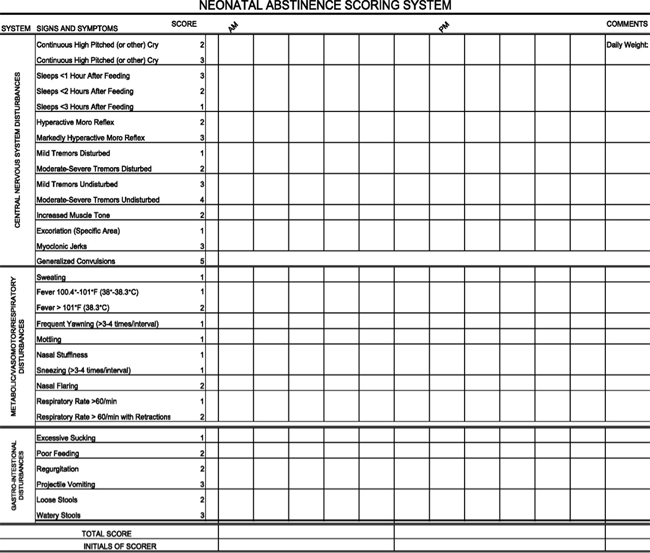
Table 3. Modified Finnegan's Neonatal Abstinence Scoring Tool.
Administration of this scoring system with infants verified not to have been exposed to prenatal opiates by meconium analysis resulted in a stable median score of 2 during each of the first 3 days of life, with 95th percentile scores of 5.5 and 7 on days 1 and 2 respectively (25).
Infants at risk of NAS should be carefully monitored in the hospital for the development of signs consistent with withdrawal. The appropriate duration of hospital observation is variable and depends on a careful assessment on the maternal drug history. An infant born to a mother on a low-dose prescription opiate with a short half-life (e.g., hydrocodone; average half-life, 4 hours) may be safely discharged if there are no signs of withdrawal by 3 days of age, whereas an infant born to a mother on an opiate with a prolonged half-life (e.g., methadone) should be observed for a minimum of 5 to 7 days.
Initial treatment of infants who develop early signs of withdrawal is directed at minimizing environmental stimuli (both light and sound) by placing the infant in a dark, quiet environment; avoiding autostimulation by careful swaddling; responding early to an infant's signals; adopting appropriate infant positioning and comforting techniques (swaying, rocking); and providing frequent small volumes of hypercaloric formula or human milk to minimize hunger and allow for adequate growth. Caloric needs may be as high as 150 to 250 cal/kg per day because of increased energy expenditure and loss of calories from regurgitation, vomiting, and/or loose stools (26). The infant needs to be carefully observed to recognize fever, dehydration, or weight loss promptly. The goals of therapy are to ensure that the infant achieves adequate sleep and nutrition to establish a consistent pattern of weight gain and begins to integrate into social environment. Maternal screening for comorbidities, such as HIV or hepatitis C virus infections and polydrug abuse, needs to be performed. Additional supportive care in the form of intravenous fluids, replacement electrolytes, and gavage feedings may be necessary to stabilize the infant's condition in the acute phase and obviate the need for pharmacologic intervention.
Breastfeeding or the feeding of human milk has been associated with less severe NAS that presents later and less frequently requires pharmacologic intervention. When possible, and if not otherwise contraindicated, mothers who adhere to a supervised drug treatment program should be encouraged to breastfeed so long as the infant continues to gain weight. Methadone is present in exceptionally low concentrations in milk. Cumulative daily intake of methadone in fully breastfed infants has been estimated to range from 0.01 to 0.15 mg/day in the first 30 days of life, and 0.15 to 0.30 mg/day between 30 and 180 days of age (27). The amount of buprenorphine excreted in human milk is small and there is no clear reason to discourage breastfeeding in mothers who adhere to methadone and buprenorphine maintenance treatment.
Pharmacologic Treatment of NAS
Drug therapy is indicated to relieve moderate to severe signs of NAS and to prevent complications such as fever, weight loss, and seizures in an infant does not respond to committed program of non-pharmacologic support. Withdrawal for opioids or sedative-hypnotic drugs may be life-threatening, but ultimately, drug withdrawal is a self-limited process. Unnecessary pharmacologic treatment will prolong drug exposure and the duration of hospitalization to the possible detriment of maternal-infant bonding. The only clearly defined benefit of pharmacologic treatment is the short-term amelioration of clinical signs.
Clinicians have treated NAS with a variety of drug preparations, including opioids (tincture of opium, neonatal morphine solution, methadone, and paregoric), barbiturates (phenobarbital), benzodiazepines (diazepam, lorazepam), clonidine, and phenothiazines (chlorpromazine). 94% of UK and 83% of US clinicians use and opioid (morphine or methadone) as the drug of first choice. The majority of practitioners use phenobarbital as a second drug if the opiate does not adequately control withdrawal signs. Daily dose of morphine ranges from 0.24 mg/kg per day to 1.3 mg/kg per day (26). Paregoric is no longer used, because it contains variable concentrations of other opioids, as well as toxic ingredients such as camphor, anise oil, alcohol and benzoic acid. The use of diazepam has also fallen into disfavor because of a documented lack of efficacy compared with other agents and because of its adverse effects on infant suck and swallow reflexes.
One of the cornerstones in caring for critically ill children is to provide adequate and safe analgesia, sedation, amnesia, and anxiolysis by suing both pharmacologic and non-pharmacologic measures. Pharmacologic treatment typically includes medications in the opioids and benzodiazepine drug classes. However, if these drugs cannot safely be discontinued within a few days, physical dependence on 1 or both of these classes of medication can develop and manifest with signs and symptoms of withdrawal on acute dosage reduction or cessation of therapy. Infants who undergo complex surgery, who require prolonged medical intensive care for conditions such as respiratory failure or persistent pulmonary hypertension, or who are supported with extracorporeal membrane oxygenation (ECMO) therapy are among those at greatest risk of acquired drug dependency.
A randomized comparison trial of sublingual buprenorphine versus neonatal opium solution for the treatment of NAS showed a non-significant reduction in length of treatment and duration of hospitalization in the buprenorphine group (28). Buprenorphine therapy was well tolerated.
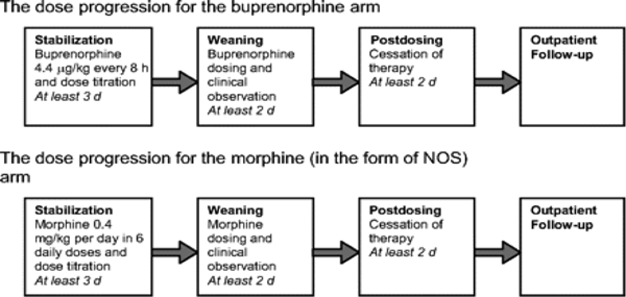
Figure 2. Study Flow (reference # 28). Abbreviation: NOS (Neonatal Opium Solution).
Standard-of-care in most teaching hospitals in USA consist of an initial dose of morphine of 0.4 mg/kg per day (in the form of NOS [neonatal opium solution]) in 6 divided doses with dose escalation of 10% per day for Finnegan scale scores >24 total on either 2 or 3 measures or a single score of 12. The final alcohol concentration of NOS usually is 0.19%. Adjunctive treatment with phenobarbital is initiated when the dose of NOS reaches 1 mg/kg per day. Infants are weaned from NOS once they demonstrated control of their NAS as measured by the Finnegan scale for 48 hours. Daily dose was weaned by 10% every 24 hours as tolerated. When an infant required rescue therapy, the equivalent of 1 extra dose of NOS is given. Regardless of treatment allocation to buprenorphine or NOS, all patients were observed for at least 2 days after the cessation of dosing. Dosing for all patients was based on birth weight (Table 4). Buprenorphine administered via the sublingual route is feasible, safe, and novel treatment for NAS.
At this time, no optimal pharmacologic regimen for the prevention or treatment of acquired opioid and/or benzodiazepine dependency can be recommended, because the necessary comparative studies of safety and efficacy are not available (29). Hence, it is even more incumbent on the practitioner to prescribe pharmacologic interventions with the goal of achieving the desired therapeutic effect by using the fewest drugs at the lowest doses and for the shortest duration possible.
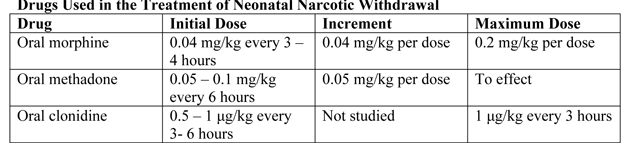
Table 4. Dosing regimens commonly used for NAS.
Clonidine is an α2-adrenergic receptor agonist that has been used in combination with an opioid or other drug in older children and adults to reduce withdrawal symptoms. Via a negative feedback mechanism, clonidine reduces CNS sympathetic outflow and palliates symptoms of autonomic overactivity such as tachycardia, hypertension, diaphoresis, restlessness, and diarrhea. Cessation of clonidine treatment can result in a rebound of autonomic activity. Reported experience with clonidine as a primary or adjunctive treatment of NAS is limited but promising.
Understanding the Full Impact of Prenatal Opioid Exposure
Although NAS is a well-recognized consequence of prenatal opioid exposure, the full impact of exposure to these psychoactive medications on the developing fetus and particularly fetal brain is unknown; the effects might extend far beyond diagnoses that are apparent in the first days and weeks of life. Of significant concern is that polysubstance exposure during pregnancy can include alcohol, a known human teratogen. The lessons learned for prenatal alcohol exposure might be relevant for opioids. Prescription opioid exposure in early pregnancy has been associated with several structural birth defects, including gastroschisis, and possible autism (30).
More research is needed to better understand the possible role of opioid exposure or related substance exposure on the occurrence of gastroschisis and other birth defects and the underlying mechanism for these potential effects. Current surveillance efforts are limited to monitoring prenatal opioid use, opioid use disorder at delivery, and diagnosis of NAS; these efforts lack information on the full range of outcomes that could be obtained from longitudinal mother-infant linked surveillance approaches.
Some preliminary data suggest that infants with a diagnosis of NAS are more likely to need special education services in preschool and early school age than infants without this diagnosis, but better data are needed to clarify the nature of the development problems and how they might relate to in-utero exposure to opioids and other substances as well as the postnatal environment (31).
Prevention and Opportunities
Obstetrics is unique in that opioid use and abuse disorders affect two patients simultaneously (the mother and fetus), and the treatment options are somewhat at odds in that they need to balance a stable maternal status and intrauterine environment with the risk of NAS. Additionally pregnancy is an opportunity for a woman with opioid use disorder to have access to medical care (possibly for the first time) leading to the diagnosis and treatment of her disease.
Screening
- Universally screen for substance use, starting at the first prenatal visit: this is recommended over risk factor-based screening.
- Use a validated screening tool. A tool such as a questionnaire is recommended as the first-line screening test (for example the 4 Ps screen, the National Institute on Drug Abuse Quick Screen, and the CRAFFT Screening Interview).
- Do not universally screen urine and hair for drugs. This type of screening has many limitations, such as the limited number of substances tested, false-positive results, and inaccurate determination of the frequency or timing of drug use. Information regarding the consequences of the test must be provided, and patient consent must be obtained prior to performing the test.
Prenatal Care
- Screen for comorbid conditions such as sexually transmitted infections, other medications or substance use, social conditions, and mental health disorders.
- Perform ultrasonography serially to monitor fetal growth because of the increased risk of fetal growth restriction.
- Consult with anesthesiology for pain control recommendations for labor and delivery and with neonatology/pediatrics for NAS counseling.
Management during pregnancy
- Use medication-assisted treatment with buprenorphine or methadone, which is preferred to medically supervised withdrawal. Medication-assisted treatment prevents withdrawal symptoms and cravings, decreases the risk of relapse, improves compliance with prenatal care and addiction treatment programs, and leads to better obstetric outcomes (higher birth weight, lower rate of preterm birth, lower prenatal mortality).
- Know that buprenorphine has several advantages over methadone, including the convenience of an outpatient prescription, a lower risk of overdose, and improved neonatal outcomes (higher birth weight, lower doses of morphine to treat NAS, shorter treatment duration).
- Prioritize methadone as the preferred option for pregnant women who are already receiving methadone treatment (changing to buprenorphine may precipitate withdrawal), those with a long-standing history of or multi-substance abuse, and those who have failed other treatment programs (32).
Intrapartum/Postpartum Care
- Recognize heightened pain. Women with opioid use disorder have increased sensitivity to painful stimuli.
- Continue the maintenance dose of methadone or buprenorphine throughout hospitalization, with short-acting opioids added for a brief period for postoperative pain.
- Prioritize regional anesthesia for pain control in labor or for cesarean delivery.
- Consider alternative therapies such as regional blocks, non-opioid medications (non-steroidal anti-inflammatory drugs, acetaminophen), or relaxation/mindfulness training.
- Avoid mixed antagonist and agonist narcotics (butorphanol, nalbuphine, pentazocine) as they may cause acute withdrawal.
- Encourage breastfeeding to decrease the severity of NAS and maternal stress and increase maternal-child bonding and maternal confidence.
- Offer contraceptive counseling and services immediately postpartum in the hospital, with strong consideration for long-acting reversible contraception.
Opioid Prescribing Practices
Opioids are prescribed in excess post-cesarean delivery. Several recent studies have demonstrated that most women are prescribed opioids post-cesarean delivery in excess of the amount they use (median 30 - 40 tablets prescribed, median 20 tablets used) (32). The leftover opioid medication usually is not discarded and therefore is at risk for diversion or misuse. A small subset of patients will use all the opioids prescribed and feel as though they have not received enough medications.
Prescribe post-cesarean delivery opioids more appropriately by considering individual inpatient opioid requirements or a shared decision-making model. Prioritize acetaminophen and ibuprofen during breastfeeding. Whenever possible, acetaminophen and ibuprofen should be the first-line treatment for breastfeeding women, and narcotics that are metabolized by CYP2D6 should be avoided to reduce the risk to the newborn.
Summary
NAS continues to be an important clinical entity throughout much of the world. NAS leads to a constellation of signs and symptoms involving multiple systems. The pathophysiology of NAS is not completely understood. Urine or meconium confirmation may assist the diagnosis and management of NAS. The Finnegan scoring system is commonly used to assess the severity of NAS; scoring can be helpful for initiating, monitoring, and terminating treatment in neonates. Non-pharmacological care is the initial treatment option, and pharmacological treatment is required in an improvement is not observed after non-pharmacological measures or if the infant develops severe withdrawal. Morphine is the most commonly used drug in the treatment of NAS secondary to opioids. An algorithmic approach to the management of infants with NAS is suggested. Breastfeeding is not contraindicated in NAS, unless mother is taking street drugs, is involved in polydrug abuse, or is infected with HIV. Future studies are required to assess the long-term effects of NAS on children after prenatal exposure.
Suggested Reading
- The Diseases of Addiction: Opiate Use and Dependence
- Fetal Alcohol syndrome: Recognition and Prevention
http://www.womenshealthsection.com/content/gynmh/gynmh013.php3
http://www.womenshealthsection.com/content/obsmd/obsm012.php3
References
- Jilani AM, Meghan TF, Pepin D, Jewell T, et al. Evaluation of State-Mandated reporting of Neonatal Abstinence Syndrome – six states, 2013 – 2017. Morbidity and Mortality Weekly Report (MMWR) 2019;68(1):6-10
- Winkelman TNA, Villapiano N, Kozhimannil KB, et al. Incidence and costs of neonatal abstinence syndrome among infants with Medicaid. Pediatrics 2018;141:e2017-3520
- Hedegaard H, Minino AM, Warner M. Drug overdose deaths in the United States, 1999 – 2017. NCHS Data Brief, No 329, Hyattsville, MD: National Center for Health Statistics; 2018
- Loudin S., Haas J, Payne M., et al. Identifying co-exposure to Opiates and Gabapentin during pregnancy. The Journal of Pediatrics 2020;217:196-198
- Hudak ML, Tan RC, The Committee on Drugs and the Committee on Fetus and Newborn. Neonatal Drug Withdrawal. Pediatrics 2012;129:e540-e560
- Lejeune C, Simmat-Durand L, Gourarier L, et al. Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenorphine substitution. Drug Alcohol Depend 2006;82(3):250-257
- Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med 2010;363(24):2320-2331
- Kayemba-Kay S, Laclyde JP. Buprenorphine withdrawal syndrome in newborns: a report of 13 cases. Addiction 2003;98(11):1599-1604
- Lacroix K, Berrebi A, Chaurmerliac C, et al. Buprenorphine in pregnant opioid-dependent women: first results of a prospective study. Addiction 2004;99(2):209-214
- Cleary BJ, Donnelly J, Strawbridge J, et al. Methadone dose and neonatal abstinence syndrome – systematic review and meta-analysis. Addiction 2010;105(12):2071-2084
- Liu AJ, Jones MP, Murray H, et al. Perinatal risk factors for the neonatal abstinence syndrome in infants born to women on methadone maintenance therapy. Aust N Z J Obstet Gynecol 2010;50(3):253-258
- Dashe JS, Sheffield JS, Olscher DA, et al. Relationship between maternal methadone dosage and neonatal withdrawal. Obstet Gynecol 2002;100(6):1244-1249
- Drozdick J III, Berghella V, Hill M, Kaltenbach K. Methadone trough levels in pregnancy. Am J Obstet Gynecol 2002;187(5):1184-1188
- Kuschel CA, Austerberry L, Cornwell M, et al. Can methadone concentrations predict the severity of withdrawal in infants at risk of neonatal abstinence syndrome? Arch Dis Child Fetal Neonatal Ed 2004;89(5):F390-F393
- Dysart K, Hsieh HC, Kaltenbach K, Greenspan JS. Sequela of preterm versus term infants born to mothers on a methadone maintenance program: differential course of neonatal abstinence syndrome. J Perinat Med 2007;35(4):344-346
- Seligman NS, Austerberry L, Cornwell M, Couch R, et al. Relationship between maternal methadone dose at delivery and neonatal abstinence syndrome. J Pediatr 2010;15793):428-433
- Johnson K, Gerada C, Greenough A. Treatment of neonatal abstinence syndrome. Arch Dis Child Fetal Neonatal Ed 2003;88(1):F2-F5
- Bada HS, Das A, Bauer CR, et al. Low birth weight and preterm births: etiologic fraction attributable to prenatal drug exposure. J Perinatol 2005;25(10):631-637
- Campolongo P, Trezza V, Palmery M, et al. Developmental exposure to cannabinoids causes subtle and enduring neurofunctional alterations. Int Rev Neurobiol 2009;85:117-133
- Choo RE, Huestis MA, Schroeder JR, et al. Neonatal abstinence syndrome in methadone-exposed infants is altered by level of prenatal tobacco exposure. Drug Alcohol Depend 2004;75(3):253-260
- Chasnoff IJ. Prenatal substance exposure: maternal screening and neonatal identification and management. NeoReviews 2003;4(9):e228-e235
- Vinner E, Vignau J, Thibault D, et al. Neonatal hair analysis contribution to establishing a gestational drug exposure profile and predicting a withdrawal syndrome. Ther Drug Monit 2003;25(4):421-432
- Montgomery D, Plate C, Alder SC, Jones M, et al. Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium. J Perinatol 2006;26(1):11-14
- Sarkar S, Donn SM. Management of neonatal abstinence syndrome in neonatal intensive care units: a national survey. J Perinatol 2006;26(1):15-17
- Zimmermann-Baer U, Notzili U, Rentsch K, Bucher HU. Finnegan neonatal abstinence scoring system: normal values for first 3 days and weeks 5-6 in non-addicted infants. Addiction2010;105(3):524-528
- O'Grady MJ, Hopewell J, White MJ. Management of neonatal abstinence syndrome: a national survey and review of practice. Arch Dis Child Fetal Neonatal Ed 2009;94(4):F249-F252
- Jansson LM, Choo R, Velez ML, et al. Methadone maintenance and breastfeeding in the neonatal period. Pediatrics 2008;121(1):106-114
- Kraft WK, Gibson E, Dysart K, et al. Sublingual buprenorphine for treatment of neonatal abstinence syndrome: a randomized trial. Pediatrics 2008;122(3):e601-e607
- Simons SH, Anand KJ. Pain Control: opioid dosing, population kinetics and side-effects. Semin Fetal Neonatal Med 2006;11(4):260-267
- Rubenstein E, Young JC, Croen LA, et al. Brief report: maternal opioid prescription from preconception through pregnancy and the odds of autism spectrum disorder and autism features in children. J Autism Dev Disord 2019;49:376-382
- Honein MA, Boyle C, Redfield RR. Public health surveillance of prenatal opioid exposure in mothers and infants. Pediatrics 2019;143(3):e20183801
- Reddy UM, Davis JM, Ren Z, Greene MF. Opioid use in pregnancy. Neonatal Abstinence Syndrome, and Childhood Outcomes Workshop Invited Speakers; Executive summary of a joint workshop. Obstet Gynecol 2017;130(1):10-28
Published: 5 August 2020
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


