Community Acquired Pneumonia in Pregnancy
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Although morbidity and mortality from pneumonia has decreased since 1901, pneumonia in pregnancy remains a major health issue worldwide. Pneumonia in the pregnant and nonpregnant population is a common disease, with considerable morbidity and mortality. Pneumonia classification includes: community acquired pneumonia (CAP) encountered in otherwise healthy individuals, health care-associated pneumonia (HCAP) developing in outpatient-care facilities, hospital-acquired pneumonia, nursing-home-acquired pneumonia, and ventilator-associated pneumonia (1). Improving the care of adult patients with CAP has been the focus of many different organizations, and several have developed guidelines for management of CAP. Two of the most widely referenced are those of the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS). In response to confusion regarding differences between their respective guidelines, the IDSA and the ATS convened a joint committee to develop a unified CAP guideline document. Enthusiasm for developing these guidelines derives, in large part, from evidence that previous CAP guidelines have led to improvement in clinically relevant outcomes. Consistently beneficial effects in clinically relevant parameters followed the introduction of a comprehensive protocol that increased compliance with published guidelines. The first recommendation, therefore, is that CAP management guidelines be locally adapted and implemented. Documented benefits are: CAP guidelines should address a comprehensive set of elements in the process of care rather than a single element in isolation; and development of local CAP guidelines should be directed toward improvement in specific and clinically relevant outcomes. Understanding the relationship between hospital volume and mortality for medical conditions in USA is critical for clinicians and policy makers, since they are under increasing pressure to identify strategies to improve the quality of care. In addition, because three of the most common and costly reasons for hospital admission among Medicare beneficiaries are acute myocardial infection, heart failure and pneumonia, identifying factors associated with better quality of care has great significance. To better understand the relationship between hospital volume and patient mortality, many studies have examined whether admission to higher-volume hospitals is associated with a reduction in 30-day mortality for Medicare beneficiaries who were hospitalized for myocardial infarction, heart failure or pneumonia.
The purpose of this document is to focus on community acquired pneumonia (CAP) in pregnancy. Recent recommendations by the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) on the management of CAP address diagnostic techniques and management schemes for bacterial and viral pneumonias are addressed. These guidelines are discussed in the setting of the pregnant woman with CAP. It is widely held that pregnant women do not tolerate lung infections as well, and thus pneumonia can result in greater morbidity and mortality. Because of this, most recommend that a higher level of surveillance and intervention be practiced for the pregnant women.
Background
There are approximately 4 million cases of CAP in the United States each year, resulting in about 1 million hospitalizations. Inpatient management of pneumonia is more than 20 times as expensive as outpatient care and costs an estimated $9 billion a year (1)(2). The efforts at improvement in care are warranted, because CAP, together with influenza, remains the seventh leading cause of death in the United States (2). According to one estimate, 915,900 episodes of CAP occur in adults 65 years of age each year in the United States. Despite advances in antimicrobial therapy, rates of mortality due to pneumonia have not decreased significantly since penicillin became routinely available. Pneumonia is a relatively common complication in pregnancy and accounts for 4.2% of antepartum admissions for non-obstetric complications (2). Fortunately, over the past 50 years, mortality rates for CAP in pregnant women have decreased from about 20% to less than 4% with appropriate antimicrobials and intensive care services (3). In the United States, the incidence of antepartum CAP averages about 1 per 1,000 pregnancies and ranges from 0.5 to 1.5 per 1,000 depending on the population studied (3). Most recommend that a higher level of surveillance and intervention be practiced for the pregnant women. Community-acquired methicillin-resistant S aureus influenza-associated pneumonia has been reported to have a fatality rate of 25% (9).
n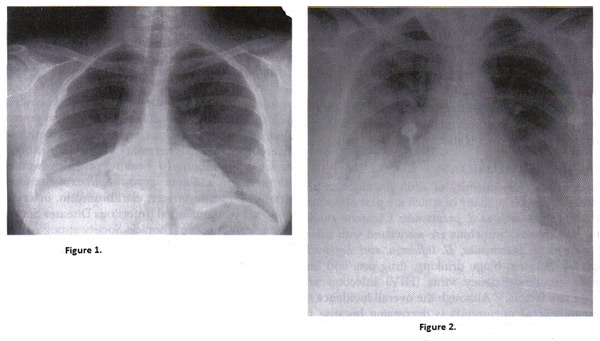
Figure 1. Chest radiograph of right pneumococcal pneumonia; Figure 2. Chest radiograph of pregnant woman with varicella pneumonia. Note the nodular and interstitial infiltrates similar to other viral pneumonias.
Most Common Etiologies of Community-Acquired Pneumonia (CAP)
In most cases, CAP develops when an infectious agent gains access to the lower respiratory tract by inhalation of aerosolized material or aspiration of upper airway microbes. Less commonly, there can be hematogenous spread. The host defense system, including anatomic and mechanical barriers and innate and humoral immunity, is overwhelmed by a combination of factors such as virulence of the pathogen, impairment of the host defense, and large bacterial inoculums. Comorbid conditions such as asthma, cigarette smoking, malnutrition, liver disease, chronic obstructive pulmonary disease, and pregnancy increase the susceptibility to complications. Once the infectious agent reaches the lower respiratory tract, direct lung injury and interstitial inflammation results, leading to intrapulmonary shunting and hypoxia. The vast array of organisms that can cause pneumonia in pregnancy (listed below). Most of these are encountered rarely, but the causative agent is identified in only 40-60% of CAP (4). In adults, 60-80% of CAP is caused by bacteria, 10-20% is atypical, and 10-15% is viral. The most common single pathogen is Streptococcus pneumoniae, which is responsible for 30-50% of identified cases. This is followed by Haemophilus influenza and Mycoplasma pneumoniae.
| Patient Type | Etiology |
|---|---|
| Outpatient Type | Streptococcus pneumoniae Mycoplasma pneumoniae Haemophilus influenzae Chlamydophila pneumoniae Respiratory viruses (Influenza A and B, adenovirus, respiratory syncytial virus, and parainfluenza). |
| Inpatient (non-ICU) | S. pneumoniae M. pneumoniae C. pneumoniae H. influenzae Legionella species Aspiration Respiratory viruses (Influenza A and B, adenovirus, respiratory syncytial virus, and parainfluenza) |
| Inpatient Intensive Care Unit (ICU) | S. pneumoniae Staphylococcus aureus Legionella species Gram-negative bacilli H. influenzae |
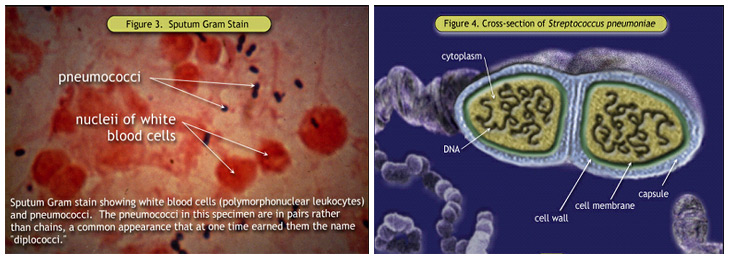
Figure 3. Sputum Gram Stain; Figure 4. Cross-section of Streptococcus pneumoniae
Certain adaptation in immune function during pregnancy also may play a role in a susceptibility to pneumonia and in its clinical course. Cytotoxic T-cells are suppressed, and T-helper type 2 cells predominate over T-helper type 1 cells by a 4:1 ratio, which results in a decrease in secretion of interleukin-2, interferon-γ, and tumor necrosis factor-β. Natural killer cell activity is also decreased (5). Although the suppression of specific humoral and cell-mediated immunologic functions is thought to allow accommodation or tolerance of the fetal graft, any possibly increased susceptibility to certain infections is clearly undesirable.
Criteria for Severe Community-Acquired Pneumonia (CAP)
Several criteria have been proposed to define severe CAP. Most case series have defined it simply as CAP that necessitates intensive care unit (ICU) admission. Objective criteria to identify patients for ICU admission include the initial ATS definition of severe CAP, and its subsequent modification, the CURB (confusion, uremia, respiratory rate, blood pressure) criteria, and Pneumonia Severity Index (PSI) severity class V (or IV and V) (6). The PSI is based on data that are commonly available at presentation and stratifies patients into five risk classes in which 30-day mortality rates range from 0.1% to 27.0%. The higher the score, the higher the risk of death, admission to the ICU, and readmission and the longer the length of stay. An algorithm that uses the PSI to judge the appropriateness of admission is helpful in the management of patients. Patients in risk classes I, II, and III are at low risk for death and most can be safely treated as outpatients in the absence of extenuating circumstances.
Minor criteria*: Respiratory rate** >30 breaths/min; PaO2/FiO2 ratio**<250; multilobar infiltrates; confusion/disorientation; uremia (BUN level, >20 mg/dL); leukopenia*** (WBC count, <4000 cells/mm3); thrombocytopenia (platelet count, <100,000 cells/mm3); hypothermia (core temperature, <36°C); hypotension requiring aggressive fluid resuscitation.
Major criteria: Invasive mechanical ventilation; septic shock with the need for vasopressors
NOTE: BUN, blood urea nitrogen; PaO2/FiO2, arterial oxygen pressure/fraction of inspired oxygen; WBC, white blood cell.
* Other criteria to consider include hypoglycemia (in nondiabetic patients), acute alcoholism/alcoholic withdrawal, hyponatremia, unexplained metabolic acidosis or elevated lactate level, cirrhosis, and asplenia.
**A need for noninvasive ventilation can substitute for a respiratory rate 130 breaths/min or a PaO2/FiO2 ratio <250.
***As a result of infection alone.
Evaluation of Community-Acquired Pneumonia (CAP)
The evaluation of CAP is initiated based on patient symptoms. Symptoms of bacterial pneumonia in pregnancy are the same as in nonpregnant individuals. Mild upper respiratory complaints precede symptoms that include cough in more than 90%, sputum production in 66%, dyspnea in 66%, and pleuritic chest pain in 50% (7). Non-respiratory symptoms including headache, fatigue, myalgias, sweats, and nausea. Viral and fungal pneumonias may present similarly depending on specific causes. Physical examination should include vital-sign determination to assess fever and intravascular volume status. An increased respiratory rate, cyanosis, use of accessory muscles, nasal flaring, and sterna retractions are all markers of respiratory distress. Thoracic examination may reveal evidence of consolidation with dullness to percussion, E to A changes, and bronchial breath sounds consistent with bacterial pneumonia. Women with viral or mycoplasmal infections often have a normal examination. Physical examination alone has a very low sensitivity and specificity for the diagnosis of pneumonia, but, used in conjunction with history, chest radiograph, and laboratory diagnosis, it remains one of the cornerstones of diagnosis.
In addition to a constellation of suggestive clinical features, a demonstrable infiltrate by chest radiograph or other imaging technique, with or without supporting microbiological data, is required for the diagnosis of pneumonia. A chest radiograph is required for the routine evaluation of patients who are likely to have pneumonia, to establish the diagnosis and to aid in differentiating CAP from other common causes of cough and fever, such as acute bronchitis. Chest radiographs are sometimes useful for suggesting the etiologic agent, prognosis, alternative diagnoses, and associated conditions. Rarely, the admission chest radiograph is clear, but the patient's toxic appearance suggests more than bronchitis. CT scans may be more sensitive, but the clinical significance of these findings when findings of radiography are negative is unclear. For patients who are hospitalized for suspected pneumonia but who have negative chest radiography findings, it may be reasonable to treat their condition presumptively with antibiotics and repeat the imaging in 24--48 hours. Microbiological studies may support the diagnosis of pneumonia due to an infectious agent, but routine tests are frequently falsely negative and are often nonspecific. A history of recent travel or endemic exposure, if routinely sought, may identify specific potential etiologies that would otherwise be unexpected as a cause of CAP.
Recommended Diagnostic Tests for Etiology: Patients with CAP should be investigated for specific pathogens that would significantly alter standard (empirical) management decisions, when the presence of such pathogens is suspected on the basis of clinical and epidemiologic clues. The need for diagnostic testing to determine the etiology of CAP can be justified from several perspectives. The primary reason for such testing is if results will change the antibiotic management for an individual patient. The spectrum of antibiotic therapy can be broadened, narrowed, or completely altered on the basis of diagnostic testing. The alteration in therapy that is potentially most beneficial to the individual is an escalation or switch of the usual empirical regimen because of unusual pathogens (e.g., endemic fungi or Mycobacterium tuberculosis) or antibiotic resistance issues. Broad empirical coverage, such as that recommended in these guidelines, would not provide the optimal treatment for certain infections, such as psittacosis or tularemia. Increased mortality (8) and increased risk of clinical failure are more common with inappropriate antibiotic therapy. Management of initial antibiotic failure is greatly facilitated by an etiologic diagnosis at admission. Urinary antigen tests (UAT) are commercially available and have been cleared by the US Food and Drug Administration (FDA) for detection of S. pneumoniae and L. pneumophila serogroup 1(9). UAT testing appears to have a higher diagnostic yield in patients with more severe illness.
Clinical indications for more extensive diagnostic testing (9):
| Indication | Blood Culture | Sputum Culture | Legionella UAT | Pneumococcal UAT | Other |
|---|---|---|---|---|---|
| ICU Admission | X | X | X | X | Xa |
| Failure of outpatient antibiotic therapy | X | X | X | ||
| Cavitary infiltrates | X | X | Xb | ||
| Leukopenia | X | X | |||
| Active alcohol abuse | X | X | X | X | |
| Chronic severe liver disease | X | X | |||
| Severe obstructive/structural lung disease | X | ||||
| Asplenia (anatomic or functional) | X | X | |||
| Recent travel (within past 2 weeks) | X | Xc | |||
| Positive Legionella UAT result | Xd | NA | |||
| Positive pneumococcal UAT result | X | X | NA | ||
| Pleural effusion | X | X | X | X | Xe |
NA, not applicable; UAT, urinary antigen test.
- Endotracheal aspirate if intubated, possibly bronchoscopy or nonbronchoscopic bronchoalveolar lavage.
- Fungal and tuberculosis cultures.
- See guidelines by WHO and CDC for details. (http://www.cdc.gov and http://www.who.int )
- Special media for Legionella.
- Thoracentesis and pleural fluid cultures.
Risk Stratification and the Decision to Hospitalize
About 30% to 50% of patients who are hospitalized with pneumonia have low-risk cases, many of which could potentially be managed at home (7). Our general policy is to hospitalize pregnant women with pneumonia for at least a 23-hour observation. Evaluation includes a chest X-ray, complete blood count, electrolytes, assessment of oxygenation, and blood cultures if clinical sepsis is suspected. For all patients, blood cultures are positive in only 5-15%; positive blood cultures are more common in those with severe CAP (9). Gram stain of sputum may be useful to choose initial antibiotic coverage; however, it usually has a low yield (10). During influenza season, rapid serologic testing for influenza A and B is reasonable. Sputum cultures, serologic testing, cold agglutinin identification, bacterial antigen testing, and polymerase chain reaction are performed only when clinically indicated.
Intensive Care Unit (ICU) Admission Decision
Direct admission to an ICU is required for patients with septic shock requiring vasopressors or with acute respiratory failure requiring intubation and mechanical ventilation. Direct admission to an ICU or high-level monitoring unit is recommended for patients with 3 of the minor criteria for severe CAP listed above. The second-level admission decision is whether to place the patient in the ICU or a high-level monitoring unit rather than on a general medical floor. Approximately 10% of hospitalized patients with CAP require ICU admission, but the indications vary strikingly among patients, physicians, hospitals, and different health care systems. Some of the variability among institutions results from the availability of high-level monitoring or intermediate care units appropriate for patients at increased risk of complications. Because respiratory failure is the major reason for delayed transfer to the ICU, simple cardiac monitoring units would not meet the criteria for a high-level monitoring unit for patients with severe CAP. One of the most important determinants of the need for ICU care is the presence of chronic comorbid conditions (11). However, approximately one-third of patients with severe CAP were previously healthy.
The rationale for specifically defining severe CAP is 4-fold:
- Appropriate placement of patients optimizes use of limited ICU resources.
- Transfer to the ICU for delayed respiratory failure or delayed onset of septic shock is associated with increased mortality (9). Although low-acuity ICU admissions do occur, the major concern is initial admission to the general medical unit, with subsequent transfer to the ICU. As many as 45% of patients with CAP who ultimately require ICU admission were initially admitted to a non-ICU setting. Many delayed transfers to the ICU represent rapidly progressive pneumonia that is not obvious on admission. However, some have subtle findings, including those included in the minor criteria (discussed above), which might warrant direct admission to the ICU.
- The distribution of microbial etiologies differs from that of CAP in general (10), with significant implications for diagnostic testing and empirical antibiotic choices. Avoidance of inappropriate antibiotic therapy has also been associated with lower mortality.
- Patients with CAP appropriate for immunomodulatory treatment must be identified (11). The systemic inflammatory response/severe sepsis criteria typically used for generic sepsis trials may not be adequate when applied specifically to severe CAP. For example, patients with unilateral lobar pneumonia may have hypoxemia severe enough to meet criteria for acute lung injury but not have a systemic response.
Antibiotic Treatment
Empirical antimicrobial therapy: Empirical antibiotic recommendations have not changed significantly from those in previous guidelines. Increasing evidence has strengthened the recommendation for combination empirical therapy for severe CAP. Only 1 recently released antibiotic has been added to the recommendations: ertapenem, as an acceptable b-lactam alternative for hospitalized patients with risk factors for infection with gram-negative pathogens other than Pseudomonas aeruginosa (12). At present, the committee is awaiting further evaluation of the safety of telithromycin by the US Food and Drug Administration (FDA) before making its final recommendation regarding this drug. Recommendations are generally for a class of antibiotics rather than for a specific drug, unless outcome data clearly favor one drug. Because overall efficacy remains good for many classes of agents, the more potent drugs are given preference because of their benefit in decreasing the risk of selection for antibiotic resistance.
Outpatient treatment
- Previously healthy and no risk factors for drug-resistant S. pneumoniae (DRSP) infection:
- A macrolide (azithromycin, clarithromycin, or erythromycin) (strong recommendation; level I evidence)
- Doxycycline (weak recommendation; level III evidence)
- Presence of comorbidities, such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancies; asplenia; immunosuppressing conditions or use of immunosuppressing drugs; use of antimicrobials within the previous 3 months (in which case an alternative from a different class should be selected); or other risks for DRSP infection:
- A respiratory fluoroquinolone (moxifloxacin, gemifloxacin, or levofloxacin [750 mg]) (strong recommendation; level I evidence)
- A b-lactam plus a macrolide (strong recommendation; level I evidence) (High-dose amoxicillin [e.g., 1 g 3 times daily] or amoxicillin-clavulanate [2 g 2 times daily] is preferred; alternatives include ceftriaxone, cefpodoxime, and cefuroxime [500 mg 2 times daily]; doxycycline [level II evidence] is an alternative to the macrolide)
- In regions with a high rate (>25%) of infection with high-level (MIC, >16 mg/mL) macrolide-resistant S. pneumoniae, consider the use of alternative agents, including those without comorbidities. (Moderate recommendation; level III evidence)
Inpatient, non-ICU treatment
- A respiratory fluoroquinolone (strong recommendation; level I evidence).
- A b-lactam plus a macrolide (strong recommendation; level I evidence) (Preferred b-lactam agents include cefotaxime, ceftriaxone, and ampicillin; ertapenem for selected patients; with doxycycline [level III evidence] as an alternative to the macrolide. A respiratory fluoroquinolone should be used for penicillin-allergic patients.) Increasing resistance rates have suggested that empirical therapy with a macrolide alone can be used only for the treatment of carefully selected hospitalized patients with non-severe disease and without risk factors for infection with drug-resistant pathogens. However, such monotherapy cannot be routinely recommended (13).
Inpatient, ICU treatment
- A b-lactam (cefotaxime, ceftriaxone, or ampicillin-sulbactam) plus either azithromycin (level II evidence) or a fluoroquinolone (level I evidence) (strong recommendation) (For penicillin-allergic patients, a respiratory fluoroquinolone and aztreonam are recommended).
- For Pseudomonas infection, use an antipneumococcal, antipseudomonal b-lactam (piperacillin-tazobactam, cefepime, imipenem, or meropenem) plus either ciprofloxacin or levofloxacin (750-mg dose) (14).
Or
The above b-lactam plus an aminoglycoside and azithromycin.
Or
The above b-lactam plus an aminoglycoside and an antipneumococcal fluoroquinolone (for penicillin-allergic patients, substitute aztreonam for the above b-lactam). (Moderate recommendation; level III evidence). - For community-acquired methicillin-resistant Staphylococcus aureus infection, add vancomycin or linezolid. (Moderate recommendation; level III evidence).
Editor's Note: Tetracycline (doxycycline, minocycline) and aminoglycoside drugs (gentamicin, tobramycin, amikacin) should be avoided during pregnancy.
Time to first antibiotic dose
- For patients admitted through the emergency department (ED), the first antibiotic dose should be administered while still in the ED. (Moderate recommendation; level III evidence). Rather than designating a specific window in which to initiate treatment, the committee felt that hospitalized patients with CAP should receive the first antibiotic dose in the ED.
Switch from intravenous to oral therapy
- Patients should be switched from intravenous to oral therapy when they are hemodynamically stable and improving clinically, are able to ingest medications, and have a normally functioning gastrointestinal tract. (Strong recommendation; level II evidence) (15).
- Patients should be discharged as soon as they are clinically stable, have no other active medical problems, and have a safe environment for continued care. Inpatient observation while receiving oral therapy is not necessary. (Moderate recommendation; level II evidence).
Duration of antibiotic therapy
- Patients with CAP should be treated for a minimum of 5 days (level I evidence), should be afebrile for 48--72 hours, and should have no more than 1 CAP-associated sign of clinical instability before discontinuation of therapy (level II evidence). (Moderate recommendation) (16).
- A longer duration of therapy may be needed if initial therapy was not active against the identified pathogen or if it was complicated by extrapulmonary infection, such as meningitis or endocarditis. (Weak recommendation; level III evidence).
Epidemiology of Non-responding Pneumonia
The term "non-responding pneumonia" is used to define a situation in which an inadequate clinical response is present despite antibiotic treatment. Lack of a clear-cut and validated definition in the literature makes non-response difficult to study. Lack of response also varies according to the site of treatment. Lack of response in outpatients is very different from that in patients admitted to the ICU. The time of evaluation is also important. Persistent fever after the first day of treatment differs significantly from fever persisting (or recurring) at day 7 of treatment. Two patterns of unacceptable response are seen in hospitalized patients (17). The first is progressive pneumonia or actual clinical deterioration, with acute respiratory failure requiring ventilatory support and/or septic shock, usually occurring within the first 72 hours of hospital admission. As is noted above, as many as 45% of patients with CAP who ultimately require ICU admission are initially admitted to a non-ICU setting and are transferred because of deterioration. Deterioration and development of respiratory failure or hypotension 172 hours after initial treatment is often related to inter-current complications, deterioration in underlying disease, or development of nosocomial superinfection. The second pattern is that of persistent or non-responding pneumonia. Non-response can be defined as absence of or delay in achieving clinical stability. When these criteria were used, the median time to achieve clinical stability was 3 days for all patients, but a quarter of patients took >6 days to meet all of these criteria for stability. Stricter definitions for each of the criteria and higher PSI scores were associated with longer times to achieve clinical stability.
Because of the limitations of diagnostic testing, the majority of CAP is still treated empirically. Critical to empirical therapy is an understanding of the management of patients who do not follow the normal response pattern. Although difficult to define, non-response is not uncommon. Overall, 6%--15% of hospitalized patients with CAP do not respond to the initial antibiotic treatment (17). The incidence of treatment failure among patients with CAP who are not hospitalized is not well known, because population-based studies are required. Almirall et al. (18) described an overall hospitalization rate of 60% in a population-based study, but the rate of failure among the 30% of patients who initially presented to their primary care physician was not provided. The frequency of prior antibiotic therapy among Medicare patients admitted to the hospital with CAP is 24%--40%, but the percentage who received prior antibiotic therapy for the acute episode of pneumonia itself versus other indications is unclear. For patients initially admitted to the ICU, the risk of failure to respond is already high; as many as 40% will experience deterioration even after initial stabilization in the ICU. Mortality among non-responding patients is increased several-fold in comparison with that among responding patients (18). Overall mortality rates as high as 49% have been reported for an entire population of non-responding hospitalized patients with CAP, and the mortality rate reported in one study of early failure was 27% (9).
Given these results, concern regarding non-response should be tempered before 72 hours of therapy. Antibiotic changes during this period should be considered only for patients with deterioration or in whom new culture data or epidemiologic clues suggest alternative etiologies. Finally, non-resolving or slow-resolving pneumonia has been used to refer to the conditions of patients who present with persistence of pulmonary infiltrates 130 days after initial pneumonia-like syndrome (18). As many as 20% of these patients will be found to have diseases other than CAP when carefully evaluated.
Management of Non-responding Pneumonia
Non-response to antibiotics in CAP will generally result in >1 of 3 clinical responses: 1) transfer of the patient to a higher level of care, 2) further diagnostic testing, and 3) escalation or change in treatment. Issues regarding hospital admission and ICU transfer are discussed above. An inadequate host response, rather than inappropriate antibiotic therapy or unexpected microorganisms, is the most common cause of apparent antibiotic failure when guideline recommended therapy is used. Decisions regarding further diagnostic testing and antibiotic change/escalation are intimately intertwined and need to be discussed in tandem. In addition to microbiological diagnostic procedures, several other tests appear to be valuable for selected patients with non-response:
- Chest computed tomography (CT). In addition to ruling out pulmonary emboli, a CT scan can disclose other reasons for antibiotic failure, including pleural effusions, lung abscess, or central airway obstruction. The pattern of opacities may also suggest alternative noninfectious disease, such as bronchiolitis obliterans organizing pneumonia.
- Thoracentesis. Empyema and parapneumonic effusions are important causes of non-response (19), and thoracentesis should be performed whenever significant pleural fluid is present.
- Bronchoscopy with bronchial alveolar lavage (BAL) and transbronchial biopsies. If the differential of non-response includes noninfectious pneumonia mimics, bronchoscopy will provide more diagnostic information than routine microbiological cultures. BAL may reveal noninfectious entities, such as pulmonary hemorrhage or acute eosinophilic pneumonia, or hints of infectious diseases, such as lymphocytic rather than neutrophilic alveolitis pointing toward virus or Chlamydophila infection. Transbronchial biopsies can also yield a specific diagnosis.
Antibiotic management of non-response in CAP has not been studied. The overwhelming majority of cases of apparent non-response are due to the severity of illness at presentation or a delay in treatment response related to host factors. Other than the use of combination therapy for severe bacteremic pneumococcal pneumonia (20), there is no documentation that additional antibiotics for early deterioration lead to a better outcome. The presence of risk factors for potentially untreated microorganisms may warrant temporary empirical broadening of the antibiotic regimen until results of diagnostic tests are available.
Prevention of Pneumonia
Specific antimicrobial prophylactic regimens are available for only a few of the large number of pathogens that may cause of pneumonia; one example is prophylaxis for Pneumocystis jirovecii (formerly carinii) pneumonia (PCP) in women with HIV-related disease. Pneumococcal vaccine, a 23-valent polysaccharide conjugate vaccine, is recommended for those pregnant women who are immunocompromised, are smokers, have alcoholism, have diabetes or cardiac, pulmonary, or renal disease, or who are asplenic (e.g., women with sickle-cell disease). The vaccine has been shown to be 60-70% effective, although it is markedly underused. This vaccine is required only once in a woman's lifetime. Primary prevention, including hand-washing and limiting contact to sick individuals, provides some protection, but no preventive measure is completely effective.
All persons >50 years of age, others at risk for influenza complications, household contacts of high-risk persons, and health care workers should receive inactivated influenza vaccine as recommended by the Advisory Committee on Immunization Practices (ACIP), Centers of Disease Control and Prevention (CDC). (Strong recommendation; level I evidence). Vaccines targeting pneumococcal disease and influenza remain the mainstay for preventing CAP. Pneumococcal polysaccharide vaccine and inactivated influenza vaccine are recommended for all older adults and for younger persons with medical conditions that place them at high risk for pneumonia morbidity and mortality (21). The new live attenuated influenza vaccine is recommended for healthy persons 5--49 years of age, including health care workers (21). Post-licensure epidemiologic studies have documented the effectiveness of pneumococcal polysaccharide vaccines for prevention of invasive infection (bacteremia and meningitis) among elderly individuals and younger adults with certain chronic medical conditions (22). The overall effectiveness against invasive pneumococcal disease among persons >65 years of age is 44%--75% (22), although efficacy may decrease with advancing age. The effectiveness of the vaccine against pneumococcal disease in immunocompromised persons is less clear, and results of studies evaluating its effectiveness against pneumonia without bacteremia have been mixed. The vaccine has been shown to be cost effective for general populations of adults 50--64 years of age and >65 years of age (21)(22). A second dose of pneumococcal polysaccharide vaccine after a >5-year interval has been shown to be safe, with only slightly more local reactions than are seen after the first dose. Because the safety of a third dose has not been demonstrated, current guidelines do not suggest repeated revaccination. The pneumococcal conjugate vaccine is under investigation for use in adults but is currently only licensed for use in young children (23). However, its use in children <5 years of age has dramatically reduced invasive pneumococcal bacteremia among adults as well (23).
Viral Pneumonia
Most cases of viral pneumonia during pregnancy are caused by influenza viruses, and these are particularly prevalent during the influenza season, which in USA is from December through March. The clinical presentation is not altered by pregnancy, with an inoculation period of 1 to 4 days after exposure. Patients are usually infectious the day before symptom onset and for 5 days thereafter. Pneumonia, whether primary viral or superimposed bacterial, is the most common complication, and it develops in about 12% of pregnant women with influenza (9). Uncomplicated influenza in pregnancy is treated with antipyretics and supportive care. Antiviral resistance patterns in recent years have fluctuated depending on influenza type. Currently, antiviral therapy is recommended for use in pregnant women with influenza. Therapy shortens the course of illness by an average of 1 day and may reduce the risk for pneumonia. The amantadines (amantadine and rimantadine) and neuraminidase inhibitors (oseltamivir and zanamivir) are the medications currently available for use in pregnancy. Primary influenza pneumonia does not respond well be antiviral therapy, and mortality remains high. Fortunately, severe primary influenza is uncommon. Superimposed bacterial pneumonia should be treated with the appropriate regimen.
Varicella-Zoster virus is the DNA virus that causes chicken pox has been reported to have had a prevalence of 0.5-0.7 per 1,000 pregnancies before widespread vaccination was begun (24). A serious and life-threatening complication is varicella pneumonia, which develops in 10% of pregnant women (24). It is a serious complication in pregnant women -- mortality rates approximate 15% even in the era of antiviral therapy. Still, this is markedly decreased compared with the mortality rate of 35-40% before the availability of antiviral agents. Management of varicella pneumonia includes treatment with intravenous acyclovir, 10 mg/kg every 5 hours (4). In addition to perinatal transmission, intrauterine infection has been documented in varicella-zoster virus-infected women with and without pneumonia. If varicella-zoster immunoglobulin is given within 96 hours of exposure to varicella, it can attenuate or prevent infection in susceptible pregnant women. In USA, the CDC recommends the use of a varicella-zoster immune globulin (VariZIG) produced in Canada and available under an expanded access protocol. The live-attenuated varicella vaccines are contraindicated in pregnancy.
Fungal and Parasitic Pneumonia
Fungal and parasitic pneumonias are opportunistic infections and are usually of greatest consequence in immunocompromised hosts, especially in women with HIV infection. Blastomycosis and histoplasmosis are the most common fungal pneumonias complicating pregnancy and are usually self-limited. Coccidioidomycosis occurs in endemic areas and may cause pneumonia. Most fungal pneumonias present similarly to viral and bacterial pneumonia. Pregnant women with complicated fungal infections, including disseminated disease, are treated with ketoconazole and amphotericin B, although safety data for long-term use in pregnancy are limited (25). With the increasing number of pregnant women with HIV infection, pneumocystis carinii pneumonia (PCP) is the leading causes of acquired immunodeficiency syndrome (AIDS)-related death among pregnant women in the US (26). The mortality rate of those with PCP is correspondingly high at about 50%. Treatment is with trimethoprim-sulfamethoxazole or pentamidine. Patients with HIV with CD4+ T-lymphocyte count of less than 200/uL, a history of oropharyngeal candidiasis, or an AIDS-related illness should receive prophylaxis (26). The preferred prophylactic regimen is trimethoprim-sulfamethoxazole, one double-strength tablet per day. Prophylaxis is 90-95% effective in preventing PCP.
Pregnancy-Related Complications and Pneumonia
Pneumonia is associated with a large range of complications in the nonpregnant individual, including meningitis, arthritis, emphysema, endocarditis, sepsis, heart failure, severe acute respiratory distress syndrome (SARS), and renal failure. These occur in similar numbers in the pregnant woman. In addition, CAP can cause a number of pregnancy-related complications. The greatest risk is preterm labor and delivery. Preterm labor rates as high as 44% have been reported in women with CAP, and preterm delivery occurs in up to a third of women (27). Some evidence indicates that preterm birth is more common when the woman with pneumonia has a comorbid condition. Recently, term and preterm rupture of membranes has been shown to be increased in women with viral and bacterial pneumonia (28). Birth weights of neonate born to women with CAP are reported to be significantly lower than average, and there is an almost two-fold increased risk for fetal growth restriction (relative risk 1.86, 95% CI 1.01-3.45) (28,29). Although fetal hemoglobin has been an increased affinity for oxygen, which favors transplacental oxygen to the fetus, marked maternal hypoxemia may lead to fetal compromise. To ensure adequate oxygenation, we attempt to maintain a maternal PaO2 of more than 65 mm Hg. Some experts have suggested delivery in the critically ill woman with CAP. This must be individualized because data are lacking as to the benefit to the woman and the fetus. The association between hospital volume and the death rate for patients who are hospitalized for acute myocardial infarction, heart failure, or pneumonia remains unclear. It is also known whether a volume threshold for such an association exists. In this recent study (30), admission to higher-volume hospital was associated with a reduction in mortality for acute myocardial infarction, heart failure, and pneumonia, although there was a volume threshold above which and increased condition-specific hospital volume was no longer significantly associated with reduced mortality.
Summary
Performance indicators are tools to help guideline users measure both the extent and the effects of implementation of guidelines. Such tools or measures can be indicators of the process itself, outcomes, or both. Deviations from the recommendations are expected in a proportion of cases, and compliance in 80%--95% of cases is generally appropriate, depending on the indicator. Four specific performance indicators have been selected for the CAP guidelines, 3 of which focus on treatment issues and 1 of which deals with prevention:
- Initial empirical treatment of CAP should be consistent with guideline recommendations. Data exist that support the role of CAP guidelines and that have demonstrated reductions in cost, mean length of stay (LOS), and mortality when the guidelines are followed. Reasons for deviation from the guidelines should be clearly documented in the medical record.
- The first treatment dose for patients who are to be admitted to the hospital should be given in the ED. Unlike in prior guidelines, a specific time frame is not being recommended. Initiation of treatment would be expected within 6--8 hours of presentation whenever the admission diagnosis is likely CAP. A rush to treatment without a diagnosis of CAP can, however, result in the inappropriate use of antibiotics with a concomitant increase in costs, adverse drug events, increased antibiotic selection pressure, and, possibly, increased antibiotic resistance. Consideration should be given to monitoring the number of patients who receive empirical antibiotics in the ED but are admitted to the hospital without an infectious diagnosis.
- Mortality data for all patients with CAP admitted to wards, ICUs, or high-level monitoring units should be collected. Although tools to predict mortality and severity of illness exist—such as the PSI and CURB criteria, respectively—none is foolproof. Overall mortality rates for all patients with CAP admitted to the hospital, including general medical wards, should be monitored and compared with severity-adjusted norms. In addition, careful attention should be paid to the percentage of patients with severe CAP, as defined in this document, who are admitted initially to a non-ICU or a high-level monitoring unit and to their mortality rate.
- It is important to determine what percentage of at-risk patients in one's practice actually receives immunization for influenza or pneumococcal infection. Prevention of infection is clearly more desirable than having to treat established infection, but it is clear that target groups are under-vaccinated. Trying to increase the number of protected individuals is a desirable end point and, therefore, a goal worth pursuing. This is particularly true for influenza, because the vaccine data are more compelling, but it is important to try to protect against pneumococcal infection as well. Coverage of 90% of adults >65 years of age should be the target.
Acknowledgement:
Gratitude expressed to Dr. Robert P. Hoffman Chairman, Infectious Diseases, Mercy Medical Center, Springfield, MA (USA) for the expert opinions, review and support in preparing this manuscript.
Suggested Reading
World Health Organization
Streptococcus pneumoniae: Initiative for Vaccine Research (IVR)
U.S. Centers for Disease Control and Prevention
Pneumonia Can Be Prevented -- Vaccines Can Help
References
- Anand N, Kollef MH. The alphabet soup of pneumonia: CAP, HAP, HCAP, NHAP and VAP. Semin Respir Crit Care Med 2009;30:3-9
- Gazmararian JA, Petersen R, Jamieson DJ, et al. Hospitalizations during pregnancy among managed care enrollees. Obstet Gynecol 2002;100:94-100
- Shariatzadeh MR, Marrie TJ. Pneumonia during pregnancy. Am J Med 2006;119:872-876
- Goodnight WH, Soper DE. Pneumonia in pregnancy. Crit Care Med 2005;33:S390-397
- File TM. Community-acquired pneumonia. Lancet 2003;362:1991--2001
- Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003;58:377--82
- Halm EA, Teirstein AS. Clinical Practice. Management of community-acquired pneumonia. N Engl J Med 2002;347:2039-2045
- Roson B, Carratala J, Fernandez-Sabe N, et al. Causes and factors associated with early failure in hospitalized patients with community-acquired pneumonia. Arch Intern Med 2004;164:502--8
- Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44(suppl):S27-72
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388--416
- Mortensen EM, Coley CM, Singer DE, et al. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med 2002;162:1059--64
- van der Eerden MM, Vlaspolder F, de Graaff CS, et al. Comparison between pathogen directed antibiotic treatment and empirical broad spectrum antibiotic treatment in patients with community acquired pneumonia: a prospective randomized study. Thorax 2005;60:672--8
- Fields BS, Benson RF, Besser RE. Legionella and Legionnaires' disease: 25 years of investigation. Clin Microbiol Rev 2002;15:506--26
- Arancibia F, Bauer TT, Ewig S, et al. Community-acquired pneumonia due to gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med 2002;162:1849--58
- Ramirez JA, Bordon J. Early switch from intravenous to oral antibiotics in hospitalized patients with bacteremic community-acquired Streptococcus pneumoniae pneumonia. Arch Intern Med 2001;161:848--50
- File TM. Community-acquired pneumonia. Lancet 2003;362:1991--2001
- Mene´ndez R, Torres A, Rodrı´guez de Castro F, et al. Reaching stability in community-acquired pneumonia: the effects of the severity of disease, treatment, and the characteristics of patients. Clin Infect Dis 2004;39:1783--90
- Almirall J, Bolibar I, Vidal J, et al. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur Respir J 2000;15:757--63
- Arancibia F, Ewig S, Martinez JA, et al. Antimicrobial treatment failures in patients with community-acquired pneumonia. Am J Respir Crit Care Med 2000;162:154--60
- Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med 2001;161:1837--42
- Harper SA, Fukuda K, Uyeki TM, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54:1--40
- US Department of Health and Human Services. Sepsis and CAP: partnerships for diagnostics development. RFA no. RFA-AI-04-043. Available at: http://grants.nih.gov/grants/guide/rfa-files/RFA-AI-04-043.html Accessed 15 August 2010
- Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 2005; 294:2043--51
- Harger JH, Ernest JM, Thurnau GR, et al. Risk factors and outcome of varicella-zoster virus pneumonia in pregnant women. J Infect Dis 2002;185:422-427
- Whitt ST, Koch GA, Fender B, et al. Histoplasmosis in pregnancy. Arch Intern Med 2004;164:454-458
- Ahmad H, Mehta NJ, Mikal VM, et al. Pneumocystis carinii pneumonia in pregnancy. Chest 2001;120:666-671
- Banhidy R, Acs N, Puho EH, et al. Maternal acute respiratory infectious diseases during pregnancy and birth outcomes. Eur J Epidemiol 2008;23:29-35
- Sheffield JS, Cunningham FG. Community-acquired pneumonia in pregnancy. Obstet Gynecol 2009;114:915-922
- Jin Y, Carriere KC, Marrie TJ, et al. The effects of community acquired pneumonia during pregnancy ending with a live birth. Am J Obstet Gynecol 2003;188:800-806
- Ross JS, Normand ST, Wang Y, et al. Hospital volume and 30-day mortality for three common medical conditions. N Engl J Med 2010;362:1110-1118
Published: 21 September 2010
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


