Toxoplasmosis: Perinatal Parasitic Infection
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Many parasitic infections are associated with significant maternal and fetal consequences if acquired during pregnancy. In general, perinatal infections have more severe fetal consequences when they occur early in gestation, because first-trimester infections may disrupt organogenesis. Second and third trimester infections can cause neurologic impairment or growth disturbances. Toxoplasma gondii derives its specific name from the gondii, a North African rodent from which this protozoon was first isolated in 1908. The organism's distribution is ubiquitous. T gondii possesses the capacity to traverse species lines and establish infection not only in man's domestic animals, but in man himself. Toxoplasma exists in nature in three forms, the trophozoite, the cyst and the oocyst. The most important mode of transmission of infection to man is through the ingestion of poorly cooked meat containing encysted organisms. Human infection occurs when infected meat is ingested or when food is contaminated by cat feces, via flies, cockroaches, or fingers. Infection rates are highest in areas of poor sanitation and crowded living conditions. Stray cats and domestic cats that eat raw meat are most likely to carry the parasite. The cyst is destroyed by heat. A susceptible cat devoid of antibodies to T gondii will become infected after ingestion of food containing encysted organisms and will excrete oocysts for several weeks. Approximately 50% of felines subsequently challenged by cyst feeding will again excrete oocysts, indicating the probability that a cat may be infectious several times during a lifetime. Those cats that hunt or eat raw meat by contaminating their by fecal excretion of oocysts may constitute a potential hazard to pregnant patients.
The purpose of this document is to describe the mode of transmission of toxoplasmosis, the maternal and fetal effects, and to offer guidelines for counseling and management during pregnancy. Parasitic infections are associated with significant maternal and fetal consequences if acquired during pregnancy. Congenital toxoplasmosis and prevention are also discussed in this review. Pregnant women who acquire toxoplasmosis should be treated. Treatment of the pregnant women with acute toxoplasmosis reduces but does not eliminate the risk of congenital infection.
Epidemiology:
Toxoplasmosis is caused by the intracellular parasite Toxoplasma gondii. T gondii exists in several forms: a trophozoite, which is the invasive form, and a cyst or an oocyst, which are latent forms. The life cycle of T gondii is dependent of wild and domestic cats, which are the only host for the oocyst. The oocyst is formed in the cat intestine and subsequently excreted in the feces. Mammals, such as cows, ingest the oocyst, which is disrupted in the animal's intestine, releasing the invasive trophozoite. The trophozoite then is disseminated throughout the body, ultimately forming cysts in brain and muscle. Human infection is acquired by consuming cysts in undercooked meat of infected animals, by insect contamination of food, by contact with oocysts from the feces of infected cats (the only definitive hosts), or by contact with infected material of insects in soil (1). Infection with T gondii usually is asymptomatic, although after an incubation of 5-18 days, some non-specific symptoms may occur. Approximately 40 to 50% of adults in the United States have antibody to this organism, and the prevalence of antibody is highest in lower socioeconomic populations. The frequency of seroconversion during pregnancy is 5%, and approximately 3 in 11,000 infants show evidence of congenital infection. Clinically significant congenital toxoplasmosis occurs in approximately 1 in 8,000 pregnancies. Toxoplasmosis is more common in Western Europe, particularly France, most likely because of the practice in that country of eating rare or raw meat. More than 80% of women of childbearing age in Paris have antibody to T gondii, the incidence of congenital toxoplasmosis is about twice as frequent as in the United States (2).
Approximately 20-25% of women of childbearing age in the United States exhibit serologic evidence of previous T gondii infection. Although the prevalence of prior infection increases with age, at no time does it attain the high incidence observed for a comparable population in certain tropical countries and France. In the United States, 2-6 women per 1,000 sero-susceptible women will acquire the infection during pregnancy. Approximately one-third of the females who acquire toxoplasmosis during pregnancy transmit the infection to their offspring. The later in gestation maternal infection is acquired, the greater the probability of fetal involvement. When infection occurs the first trimester of pregnancy, approximately 14% of the offspring will be infected; the figures for infection acquired during the second and third trimesters are 29% and 59%, respectively. The earlier the infection occurs in pregnancy, the more severe the disease is in the newborn. Almost all infected infants born to mothers who acquire the infection in the third trimester will appear normal at birth and only months or years later develop any clinical manifestations of the infection.
Clinical Manifestations:
The ingested organism invades across the intestinal epithelium and spreads hematogenously throughout the body. Intracellular replication leads to cell destruction. Clinical manifestations of infection are the result of direct organ damage and subsequent immunologic response to parasitemia and cell death. Host immunity is mediated primarily through T-lymphocytes (3). Most infections in humans are symptomatic. Even in the absence of symptoms, however, patients may have evidence of multi-organ involvement, and clinical disease can follow a long period of asymptomatic infection. Symptomatic toxoplasmosis usually presents as an illness similar to mononucleosis. In contrast to infection in the immunocompetent host, toxoplasmosis can be a devastating infection in the immunosuppressed patient. Because immunity to T gondii is cell-mediated, patients with human immunodeficiency virus infection and those treated with chronic immunosuppressive therapy after organ transplantation are particularly susceptible to new or reactivated infection. In these patients, dysfunction of the central nervous system is the most common manifestation of infection. Findings typically include encephalitis, meningoencephalitis, and intracerebral mass lesions. Pneumonitis, myocarditis, and generalized lymphadenopathy also occur commonly.
Most often, toxoplasmosis presents as asymptomatic cervical lymphadenopathy, with symptoms occurring in only 10-20% of adult cases. Other symptoms include fever, malaise, night sweats, myalgias, and hepatosplenomegaly. Parasitemia can occur after infection, which in pregnant women can seed the placenta and cause subsequent fetal infection. Congenital transmission of T gondii depends on the time of acquisition of maternal infection. The later in gestation that the infection occurs, the more likely transmission is to occur. The rate of vertical transmission increases from 10% to 15% in the first trimester, to 25% in second trimester, and to more than 60% in third trimester (2). Within obstetric population, the most commonly recognized manifestation of acute toxoplasmosis is lymphadenopathy. It may be the sole presenting sign or there may be an associated febrile response. The nodal enlargement may focally involve the cervical, supraclavicular or inguinal regions, and is frequently unilateral. Nodular biopsy, in conjunction with the absence of significant lymphadenitis, accounts for the firm, non-tender, enlarged lymph nodes commonly observed. With more advanced disease, fatigue is the most common presenting symptom in more severe infection. It may be associated with headache, mental depression, myalgias, and a low-grade intermittent fever. A migratory polyarthritis and various types of predominantly macular rashes have also been described. In rare instances, abdominal pain secondary to mesenteric lymph node involvement may be the principal presenting complaint. The severest manifestations of systemic disease are myocarditis, meningoencephalitis, or both.
Diagnosis:
The diagnosis of toxoplasmosis in the mother can be confirmed by serologic and histologic methods. T gondii is most easily identified in lymphatic or brain tissue. Histologic preparations can be examined by light and electron microscopy. For light microscopy, specimens should be stained with either Giemsa's or Wright's stains (4). The principal histologic characteristic of these lymph nodes is marked reticulum cell hyperplasia. Microscopically, the nodal architecture is rather well preserved. The typical triad of the disease, which, however, is not present in all cases, is constituted by (a) marked follicular hyperplasia, associated with intense mitotic activity and phagocytosis of nuclear debris; (b) small granulomas composed almost entirely of epithelioid cells, located within the hyperplastic follicles and the periphery, encroaching on and blurring their margins; and (c) distention of marginal and cortical sinuses by monocytoid B cells. An additional feature is the presence immunoblasts and plasma cells in the medullary cords (5). Variations on the theme include presence in the granulomas of necrosis or more than an occasional Langhans' giant cell.
n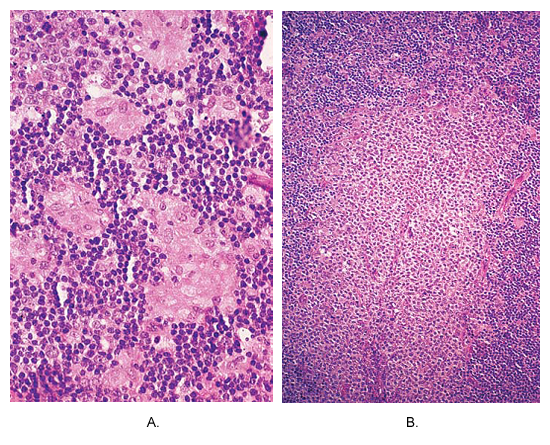
Toxoplasmosis of lymph node. (A) Small non-caseating granulomas composed of epithelioid cells are located at the periphery of hyperplastic follicle. This picture is almost pathognomonic of this disease. (B) An area of massive monocytoid B-cell hyperplasia.
Isolation of T gondii from blood or body fluids establishes that the infection is acute; however serologic testing for the detection of specific antibody to T gondii is the primary method of diagnosis. After an acute infection, IgM antibodies appear early and reach maximum levels in 1 month. IgG antibodies appear after IgM antibodies, are detectable within a few weeks after infection, and confer immunity. High titers of both IgG and IgM may persist for years. In the immunocompetent adult, the clinical course is benign and self-limiting. IgG and IgM testing should be used for the initial evaluation of patients suspected to have toxoplasmosis. Testing of serial specimens 3 weeks apart in parallel gives the most accurate assessment if the initial test results are equivocal. In cases in which clinical suspicion is high, specimens should be saved for repeat testing at the reference laboratory, because of the wide variation between laboratories.
Screening for toxoplasmosis during pregnancy:
A multicenter study in the United States found that approximately 38% of pregnant women have evidence of prior toxoplasmosis infection (7). Evidence of previous infection signifies that the future mother is not at risk of giving birth to a child with congenital toxoplasmosis. Serologic screening as a way to prevent congenital toxoplasmosis would have the most impact in countries with a high frequency of seropositivity, and routine prenatal screening is performed in France and Austria. However, in the United States, routine screening during pregnancy currently is not recommended, except in women infected with human immunodeficiency virus (HIV). Serologic screening during pregnancy may yield equivocal results, because IgM antibodies can persist for long periods. Exceptional circumstances may justify toxoplasmosis titer screening for pregnant women who are cat owners. One study in Belgium demonstrated a 63% reduction in the rate of maternal toxoplasmosis infection after institution of an educational program that recommended avoiding eating undercooked or raw meat, wearing gloves when working with soil, and avoiding caring for cats unless they are strictly "indoor cats" whose food is rigidly controlled (8).
Perinatal Assessment and Monitoring:
Ultrasonography can demonstrate severe congenital toxoplasmosis; suggestive findings include ventriculomegaly, intracranial calcifications, microcephaly, ascites, hepatosplenomegaly, and intrauterine growth restriction. Testing fetal blood samples after 20 weeks of gestation for the presence of specific IgM is the most sensitive test in diagnosing congenital toxoplasmosis. Using fetal blood for antibody testing or mouse inoculation, amniotic fluid for PCR, or fetal ultrasonography to detect ventriculomegaly, 77-93% of infected infants can be identified prenatally, although no single test is very sensitive (8). Successful identification of T gondii intrauterine infection with PCR testing of amniotic fluid allows for earlier testing than fetal blood sampling, with high sensitivity, although false-positive and false-negative findings do occur.
To evaluate sensitivity, specificity, and predictive values of a prenatal amniotic fluid (AF) polymerase chain reaction (PCR) test for diagnosis of congenital toxoplasmosis a multicenter prospective study was done on 271 women with proven primary toxoplasmosis infection during pregnancy and who had amniocentesis for prenatal diagnosis by PCR (12). Live-born infants were eligible for analysis only if a serologic follow-up could assess a definitive infection status.
Results: Of the 270 evaluable cases, 75 were congenitally infected, 48 of whom had a positive PCR at prenatal diagnosis. Overall sensitivity of PCR on AF was estimated at 64% (95% confidence interval [CI] 53.1%, 74.9%), negative predictive value of 87.8% (95% CI 83.5%, 92.1%), whereas specificity and positive predictive value were 100% (95% CIs 98%, 100% and 92.3%, 100%, respectively). Among cases with congenital toxoplasmosis, there were no significant differences between those with positive or negative PCR with regard to median gestational age at maternal infection, interval between maternal infection and amniocentesis, or duration of treatment before amniocentesis. However, sensitivity of PCR was found to be significantly higher for maternal infections that occurred between 17 and 21 weeks' gestation (P < .02). A negative PCR of AF cannot rule out congenital infection. In this case, continuation of treatment with spiramycin combined with ultrasonographic follow-up and postnatal follow-up are warranted. The results also suggest presumptive treatment combining pyrimethamine and sulfonamides in case of maternal infection occurring late in pregnancy.
Chronic or Recurrent Maternal Parasitemia:
Although congenital transmission of T gondii occurs in chronically infected animals, it has long been presumed that primary maternal infection with T gondii had to occur during gestation in order to involve the conceptus. This concept is no longer held to be valid. In a study 34 gravidas were cultured who exhibited serologic evidence of chronic toxoplasmosis and whose pregnancy terminated in abortion, stillbirth, or neonatal death. The investigators recovered the organism in two cases of abortion and in one case of neonatal death (10). Gravidas do not have to acquire primary infection during gestation to transmit the organism to the conceptus. Persistent parasitemia in this study occurred despite high antitoxoplasma antibody levels. Recurrent toxoplasmosis in severely immune-compromised individuals with CD4+ T-cell counts less than 50-100 cells/mm3 is a well documented phenomenon. Congenital infection in successive offspring may occur as a very rare event. Although the risk of a second infected infant appears very small, it nonetheless exists. Following the birth of a congenitally infected infant, it may be prudent for the mother to use some form of contraceptive for at least one year. The question of whether or not to administer chemotherapeutic agents to the mother is very controversial. If therapy is instituted, it is for potential fetal and not for maternal considerations.
Effect on Fetus and Newborn:
The parasite T gondii may cross the placenta in association with acute maternal infection. The organism may directly infect the fetus and has been linked to sporadic fetal death, which may occur in up to 5% of pregnancies after first trimester infection. However, the rate of primary infection is about 1 per 11,000 in the United States (9). Many cases of fetal death do not undergo adequate evaluation for possible causes. Perinatal autopsy and placental examination are perhaps the most valuable tests for the evaluation of fetal death. Antenatal surveillance and emotional support is the mainstay of subsequent pregnancy management. The relative importance of all of the infections is influenced by the local prevalence of the infectious agents (13). The severity of infection depends on gestational age at the time of transmission. The earlier the fetus is infected, the more severe the disease. Most infected infants do not have clinical signs of infection at birth, but 55-85% will develop sequelae, including chorioretinitis - leading to severe impairment of vision. Other clinical manifestations are hearing loss or mental retardation. Rash, hepatosplenomegaly, ascites, fever, periventricular calcifications, ventriculomegaly, and seizures are also seen in the newborns and infants infected by toxoplasmosis (9). The TORCH (serology for toxoplasmosis, rubella, cytomegalovirus, and herpes simplex) baby due to T gondii is usually infected during the first trimester of pregnancy. Second trimester fetal infection more probably results in formes frustes of disease, that are more common than the full-blown syndrome. These clinical presentations are unexplained hepatomegaly or hepatosplenomegaly, disseminated intravascular coagulopathy present at birth, or jaundice in the first 24 hours of life. In these instances, the serum IgM level tends to be greater than 20 mg/mL; elevation of the IgM level is a crude gauge of the chronicity of infection.
Congenital Toxoplasmosis:
Congenital infection can occur if a woman develops acute primary toxoplasmosis during pregnancy. Chronic or latent infection is unlikely to cause fetal injury except perhaps in an immunosuppressed patient. Approximately 40% of neonates born to mothers with acute toxoplasmosis show evidence of infection. Congenital infection is most likely to occur when maternal infection develops in the third trimester. Less than half of affected infants are symptomatic at birth. The clinical manifestations of congenital toxoplasmosis are varied and are summarized below (7):
- Rash;
- Hepatosplenomegaly;
- Ascites;
- Fever;
- Chorioretinitis;
- Periventricular calcification;
- Ventriculomegaly;
- Seizures;
- Mental retardation;
- Uveitis
The most valuable tests for antenatal diagnosis of congenital toxoplasmosis are ultrasound, cordocentesis, and amniocentesis. Ultrasound findings suggestive of infection include ventriculomegaly, intracranial calcifications, microcephaly, ascites, hepatosplenomegaly, and growth restriction. Fetal blood samples can be tested for IgM-specific antibody after 20 to 22 weeks' gestation. Fetal blood and amniotic fluid can be inoculated into mice, and the organism can be subsequently be recovered from the blood of infected animals. In addition, Hohlfeld et al, have now identified a specific gene of T. gondii in amniotic fluid using PCR (6). In their investigation, 34 of 339 infants had congenital toxoplasmosis confirmed by serologic testing or autopsy. All amniotic fluid specimens from affected pregnancies were positive by PCR, and test results were available within 1 day of specimen collection. In a subsequent investigation, Romand et al (12) reported that the PCR test had an overall sensitivity of 64% (95% confidence interval, 53-75%) for diagnosing congenital toxoplasmosis. No false-positive results were noted, and the positive predictive value was 100%.
Management in Pregnancy:
Toxoplasmosis in the immunocompetent adult is usually an asymptomatic or self-limited illness and does not require treatment. It is indicated when acute toxoplasmosis occurs during pregnancy. Treatment of mother reduces the risk of congenital infection and decreases the late sequelae of infection. Treatment of the pregnant woman with acute toxoplasmosis reduces but does not eliminate the risk of congenital infection (13). Identification of acute maternal infection necessitates immediate institution of treatment until results of fetal testing are known. Spiramycin, which concentrates in the placenta, may reduce the risk of fetal transmission by 60%, but as a single agent, it does not treat established fetal infection. If fetal infection is established; pyrimethamine, sulfadiazine, and leucovorin (folinic acid) are added to the regimen because they more effectively eradicate parasites in the placenta and in the fetus than spiramycin alone. With treatment, even early fetal infection with toxoplasmosis can result in successful pregnancy outcomes.
Spiramycin - 1.0 g, po every 8 hourly.
Pyrimethamine - 50-100 mg, po twice a day on 1st day, then 25 mg once a day.
Sulfadiazine - 1-1.5 g, po every 6 hourly
Leucovorin (folinic acid) - 10 mg or more/day.
Treatment is given for 1-2 weeks beyond resolution of signs/symptoms; continue leucovorin (folinic acid) 1 week after stopping pyrimethamine. For congenital toxoplasmosis, toxo-meningitis in adults and chorioretinitis add prednisone 1 mg/kg/day in 2 divided doses, until CSF concentration falls or vision-threatening inflammation has subsided. Leucovorin (folinic acid) dose is adjusted by following CBC results (14). Pyrimethamine is not recommended for use during the first trimester of pregnancy because of possible teratogenicity although this has not been reported to date. Sulfonamides can be used alone, but single-agent therapy appears to be less effective than combination therapy. In Europe, spiramycin has been used extensively in pregnancy with excellent success. It is available for treatment in the United States through the Centers for Disease Control and Prevention (CDC).
Recommendations of the World Health Organization for acute toxoplasmosis in pregnancy (10):
- Up to the end of the 20th week of gestation, nine million units of spiramycin are orally administered daily for 4 weeks. After 4 weeks this regimen is repeated.
- After 20 weeks of gestation, a 4 week course of sulfadiazine (1,000 mg/day) in combination with pyrimethamine (25 mg/day) and folinic acid (10 mg/week) are administered. After a pause of 4 weeks this regimen is repeated. A maximum of three treatment cycles can occur between 20 weeks gestation and delivery.
Immuno-compromised patients, however, should be treated, and the regimen of choice is a combination of oral sulfadiazine (4-g loading dose, then 1 g four times daily) plus pyrimethamine (50 to 100 mg initially, then 25 mg daily). In such patient, extended courses of treatment may be necessary to cure the infection (14).
Chemotherapy is indicated for individuals who have severe forms of toxoplasmosis or immunologic impairment of the host defense mechanism. The standard treatment in adults consists of pyrimethamine (Daraprim) -- 100 mg twice a day for the first day, followed by 50 mg a day thereafter, and sulfadiazine -- 1.5 g twice a day. The combination of pyrimethamine and sulfonamides is synergistic against trophozoites. The pyrimethamine -- sulfadiazine -- folinic acid regimen is alternated every three weeks with spiramycin -- 3 g daily until delivery. There is no effective therapy currently available against encysted form of T gondii. Thrombocytopenia, agranulocytosis, or megaloblastic anemia may develop as a consequence of therapy. Baker's yeast 5-7 g daily or folinic acid 10-20 mg daily should be given concurrently to obviate hematologic toxicity. Women undergoing therapy should be closely followed with leukocyte assays, platelet counts, and hematocrit determinations bi-weekly. Pyrimethamine administration can result in megaloblastic anemia and/or pancytopenia.
Sulfadiazine can cause renal failure secondary to crystallization within renal tubules and severe epidermal necrolysis. Individuals receiving this drug need to drink ample fluids and avoid dehydration. Because of concern of possible teratogenic consequences, the drug should be avoided in the first trimester in the absence of overriding maternal considerations.
Therapy for Fetal Indications:
There is reservation about instituting therapy for an asymptomatic gravida with acute infection during gestation. The indications for therapy are those of potential fetal involvement and not of maternal derivation. Only 25-35% of women whose gestation is complicated by acute toxoplasmosis will give birth to a congenitally infected neonate. In general, documented maternal infection is an indication for therapy irrespective of signs and symptoms of systemic disease. The therapeutic focus is aimed at attempting to avert or limit future organismal-cell interaction. An informed consent should be obtained which clearly states that the gravida is aware not only of the potential problems associated with drug therapy but also that she will not benefit per se from the therapy. There should be a willingness on the part of mother to share the responsibility of drug therapy. For first therapy be withheld during the period of organogenesis. Only one-third of the fetuses will actually require therapy, yet 100% of the fetuses will be subject to drug exposure during the critical periods of organogenesis. The Europeans advocate the use of spiramycin as soon as the diagnosis of maternal infection is established. Once organogenesis is completed, a combination treatment regimen using pyrimethamine, sulfadiazine, and spiramycin is implemented, because spiramycin does not reliably cross the placenta.
Sulfonamides should be discontinued two to three weeks prior to the expected date of confinement to avert the problem of competitive antagonism with bilirubin in the postpartum period. The sulfonamides successfully compete with bilirubin for the albumin binding site. Extensive displacement of bilirubin from albumin binding sites can be responsible for the induction of kernicterus in the neonate. Comparative tests have shown that sulfapyrazine, sulfamethazine and sulfamerazine are about as effective as sulfadiazine. Sulfathiazole, sulfapyridine, sulfadimetine, and sulfisoxazole are much less effective and are not recommended. The usual dosage of sulfadiazine or triple sulfonamides is 50-100 mg per kg of body weight every 24 hours in two to four equal doses by mouth.
Aggressive early treatment of infants with congenital toxoplasmosis is indicated and consists of combination therapy (14). Treatment of infants with symptomatic congenital toxoplasmosis consists of pyrimethamine and sulfadiazine, alternating monthly with spiramycin, for 1 year. Treatment will diminish or resolve intracranial calcifications if present, suggesting improved neurologic function. Early treatment reduces, but does not eliminate, the late sequelae of toxoplasmosis such as chorioretinitis.
Prevention:
Toxoplasma infection of the pregnant female is preventable. This is accomplished by avoidance of ingestion of cysts or sporulated oocysts by the seronegative woman. Cysts are rendered non-infective by heating meat at 66șC or by smoking or curing it. Freezing is less reliable since it requires temperature (-20șC) not achieved by most home freezers. Raw fruits and vegetables should be thoroughly washed and specific steps taken to prevent access to flies, cockroaches and other insects to animal feces. Hands should be washed thoroughly after handling raw meat or vegetables. The handling of cat feces should be avoided altogether. If this is not possible, disposable gloves should be worn when disposing of cat litter and when gardening out of doors. Treatment of the cat litter pan with neatly boiling water for five minute will kill potentially infective oocysts. In a recent study of risk factors, frequent contact with soil may be more important a risk for maternal infection than the household presence of a cat. The key elements in preventing maternal toxoplasmosis include cocking meat until it is well done, washing fruits and vegetables and wearing gloves when working in the garden (if cats frequent the area) or disposing of cat litter (15). There are no drugs to kill T gondii tissue cysts in human or animal tissues. Freezing to -20șC, cooking to an internal temperature of 66șC, or gamma irradiation (0.5 kGy) can kill tissue cysts in meat.
Summary:
Routine serological screening for toxoplasmosis is probably not cost effective; however, limited screening of gravidas who like raw or poorly cooked meat, who have significant contact with animals or who do extensive gardening is advocated. IgG specific antibodies will be present in a significant number of these patients. Only those gravidas with a concomitant specific IgM titer need further evaluation and management. All gravidas who are immunologically compromised or immunosuppressed should be screened for the presence of anti-toxoplasma antibodies. Seropositive women need to be carefully monitored for potential reactivation of disease. A multicenter study in the United States has found that approximately 38% of pregnant women have evidence of prior toxoplasmosis infection. Evidence of previous infection signifies that the future mother is not at risk of giving birth to a child with congenital toxoplasmosis. In the United States, routine screening during pregnancy currently is not recommended, except in women infected with human immunodeficiency virus (HIV). Pregnant women who acquire toxoplasmosis should be treated with spiramycin. When diagnosed, fetal toxoplasmosis should be treated with a combination of pyrimethamine, sulfadiazine, and folinic acid, alternating with spiramycin. The diagnosis of toxoplasmosis should be confirmed by a reliable reference laboratory.
Suggested Readings:
- World Health Organization
Report of the WHO Working Group meeting on Toxoplasmosis Vaccine Development and Technology - US Department of Health and Human Services; National Toxicology Program
Toxoplasmosis - Center for Disease Control and Prevention
Toxoplasmosis and Pregnancy - Facts and Prevention
References:
- Stray-Pedersen B. Toxoplasmosis in Pregnancy. Baillieres Clin Obstet Gynaecol 1993;7:107-137 (Level III)
- Levine EM. Risk factors for Toxoplasma gondii infection in mothers of infants with congenital toxoplasmosis: Implications for prenatal management and screening. Am J Obstet Gynecol 2006;194:589
- Egerman RS, Beazley D. Toxoplasmosis. Semin Perinatol 1998;22:332-338
- Lymph nodes. In Surgical Pathology; 9th edition. Editors: Rosai and Ackerman 2004. Publisher Mosby
- Hohlfeld P, Daffos F, Costa JM, et al. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N Engl J Med 1994;331:695-699 (Level II-2)
- Sever JL, Ellenberg H, Ley AC et al. Toxoplasmosis: maternal and pediatric findings in 23,000 pregnancies. Pediatrics 1988;82:181-192 (Level II-3)
- ACOG Practice Bulletin. Perinatal viral and parasitic infections. Number 20, September 2000
- Hill D, Dubey JP. Toxoplasmosis gondii: transmission, diagnosis and prevention. Clin Microbial Infect 2002;8:3-634-640
- Toxoplasma gondii. In Infectious diseases in obstetrics and gynecology; 5th edition. Editors: Monif GG, Baker DA; Parthenon publishing, 2004
- Bastien P. Molecular diagnosis of toxoplasmosis. Trans R Soc Trop Med Hyg 2002;96:S205-215
- Romand S, Wallon M, Franck J et al. Prenatal diagnosis using polymerase reaction on amniotic fluid for congenital toxoplasmosis. Obstet Gynecol 2001;97(2):296-300
- Goldenberg RL, Thompson C. The infectious origins of still birth. Am J Obstet Gynecol 2003;189:861-873
- Silver RM. Fetal Death. Obstet Gynecol 2007;109:153-167
- Duff P. Maternal and perinatal infections. In Obstetrics: Normal and Problem Pregnancies; 5th edition. Eds: Gabbe SG, Niebyl JR, Simpson JL. Publisher: Churchill Livingstone Elsevier; 2007
- Dean V, Coonrod BW, Jack PG et al. The clinical content of preconception care: infectious diseases in preconception care. Am J Obstet Gynecol 2008;199:S296-S309
Published: 3 September 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


