Principles of Genetic Counseling and Prenatal Diagnosis
Dr. Francis H. Boudreau
Chairman, Department of Obstetrics and Gynecology
St. Elizabeth's Medical Center, Boston, MA (USA)
In collaboration with Women's Health & Education Center (WHEC)
The availability of prenatal diagnosis for a wide range of disorders has been a major advance in the area of reproductive genetics. Ultrasound has played a central role in the development of the various approaches to prenatal diagnosis. In addition, integration of a genetics-based prenatal diagnosis program with tertiary ultrasound has been shown to increase the accuracy of diagnosis when compared with ultrasound alone.
The purpose of this document is to focus on the epidemiology of genetic defects and include a description of the various prenatal diagnostic procedures in use.
Epidemiology
All pregnancies carry a baseline risk of birth defects. Most geneticists quote a figure of 3% to 4% for major congenital malformations. It is important to keep this baseline figure in mind when counseling couples regarding their risk of a specific defect. The etiologies for the birth defects can be broken down into 4 categories:
- Chromosomal
- Single gene or Mendelian disorders
- Multifactorial disorders
- Teratogenic or environmental effects
Malformations are defects in the structure of an organ or region of the body resulting from an intrinsically abnormal process of development. Such malformations may be single or multiple; they include, for example, birth defects caused by single gene defects. Deformations are abnormalities in form, shape, or position caused by mechanical forces. Such deformations often occur prenatally as a result of intrauterine molding or constraint. Factors leading to deformations may be extrinsic or intrinsic. Disruption is a morphologic defect resulting from breakdown of previously normal tissue. Disruptions can be caused by extrinsic forces, internal interference with a developmental process or vascular insults. Examples of disruptions include amputations due to amniotic bands, and gastroschisis and porencephaly, both thought to result from in- utero vascular insults. Sequence is a pattern of multiple abnormalities resulting from a single primary anomaly or mechanical factor; it may be a malformation, deformation or disruption. An example is Potter sequence, in which oligohydroamnios from any cause leads to similar features of fetal compression: characteristic facial features, abnormal positioning of the hands and feet, and pulmonary hypoplasia. Syndrome is a pattern of multiple anomalies known to have a common, specific etiology. An example is Down syndrome, which is caused by the presence of an extra copy of chromosome 21. Association is used to identify the occurrence of two or more features more commonly than expected by chance alone but for which no etiology has been demonstrated. A common example is the VATER (Vertebral, Anal, Tracheo-Esophageal, and Renal anomalies) association. This term does not imply a specific diagnosis but rather prompts the search for specific other defects when one component of the association is identified.
I. Chromosomal Abnormalities:
Chromosomal disorders occur in approximately 0.5% of newborns and it is well known that the risk for many chromosomal abnormalities increases with advancing maternal age (1). Studies of the prenatal origin of chromosome 21 in Down syndrome have shown that 95% of cases of trisomy 21 are due to maternal nondisjunction and only about 5% of cases contain an extra paternal 21. Although maternal age is strongly correlated with risk for Down syndrome, most children with Down syndrome are born to women younger than 35 years. Many other chromosomal abnormalities are also associated with increasing maternal age, including 13 and 18; 47,XXX; 47,XXY; and 47,XYY. Structural chromosomal abnormalities, such as translocations and deletions, as well as Turner syndrome (45,X) do not appear to be age-related.
Chromosomal abnormalities are the leading known cause of pregnancy loss, and at least 95% of chromosomally abnormal conceptions are lost before term. It is estimated that a minimum of 10% to 15% of conceptions are chromosomally abnormal. Studies of miscarriages indicate that about 50% of such chromosomal abnormalities are trisomies, 20% are monosomies, 15% are triploid, and the remainder is tetraploids and structural abnormalities. Some chromosomal abnormalities, such as trisomy 16, are common at conception but never seen in live-born children.
Chromosomal Abnormalities in Liveborns (2):
| Type of Abnormality | Incidence |
|---|---|
| Numerical aberrations: Sex chromosomes 47,XYY |
1/1080 male births |
| Autosomal trisomies 13 |
1/19,000 |
| Structural Balanced abnormalities: Robertsonian t(Dq;Dq) |
1/1500 |
| Unbalanced structural abnormalities: Robertsonian |
1/14,000 |
II. Single Gene Disorders:
Single gene, or mendelian, disorders occur in approximately 1% of newborn infants. A large number of Mendelian disorders have been described, although the incidence of any specific disorder in general is rare. For example, cystic fibrosis has a carrier frequency of approximately 1 in 20, resulting in a disease frequency of approximately 1 in 1600. Of the recognized single gene traits, more than half are autosomal-dominant, about a third are autosomal-recessive, and the remainder are X-linked.
Autosomal-Recessive Disorders: Occurs most commonly in persons whose healthy parents both carry the same recessive gene. The risk to the offspring of such parents is 25% in each pregnancy, and these disorders most commonly occur with no family history. Autosomal-recessive disorders are often severe, and many are metabolic disorders. Carriers will have on average 50% of enzyme activity. The increased risk to parents who are first cousins to have a child with severe abnormalities is approximately 6% or twice the background risk.
Autosomal-Dominant Disorders: affected people are heterozygous for an abnormal allele, which they transmit to 50% of their offspring, male or female. Unaffected people do not transmit the disorder. Age of onset of many of these disorders is variable and people with a defective gene, who are therefore destined to develop the disease, may be asymptomatic until well into adulthood. Persons at risk may have children of their own before the age at which they develop symptoms. The severity of many autosomal disorders varies considerably, even within a family. The likely severity in any affected offspring is difficult to predict, and a mildly affected parent may have a severely affected child. New mutations for autosomal dominant disorders appear to be more common with increasing paternal age. Because of the large numbers of autosomal-dominant conditions that can arise from such mutations, no specific prenatal testing is available. In neurofibromatosis approximately 50% of cases are due to new mutations. In achondroplasia, the homozygous form is lethal and can be predicted to occur in 25% of cases with two affected parents. Of such offspring, 50% will be heterozygous and affected, and 25% will be normal.
X-Linked Disorders: most X-linked disorders are recessive, with hemizygous (having just one copy of the X chromosome) males affected and heterozygous female carriers unaffected. A female carrier will transmit the mutant gene to 50% of her offspring; therefore, half of her sons will be affected and half of her daughters will be carriers. An affected male will pass the mutant X chromosome to all of his daughters (who are therefore obligate carriers) and to none of his sons (who inherit only his Y chromosome). Duchenne muscular dystrophy may be severe or lethal during early life, so that affected sons do not reproduce. X-linked disorders can also be dominant, although this type of inheritance is much less common.
III. Multifactorial Inheritance:
Isolated birth defects, such as clubfoot, cleft lip, and neural tube defects (NTDs) are among the most common genetic abnormalities and are inherited through a combination of genetic and environmental factors. Examples of multifactorial traits are: hydrocephly, neural tube defects, cleft lip with or without cleft palate, cleft palate, cardiac abnormalities, diaphragmatic hernia, omphalocele, renal agenesis, Mullerian fusion defects, limb reduction defects, pyloric stenosis and talipes equinovarus.
The recurrence risk for relatives of an affected family member is typically higher than the population risk, with most multifactorial disorders having a recurrence risk of 2% to 5% in first-degree relatives. This risk is increased if more family members are affected. There is also some increased risk of multifactorial disorders with parental consanguinity. Multifactorial traits generally fall into one of the three following categories: continuously variable threshold or complex disorders. Continuous traits tend to be less extreme in the offspring of affected individuals, because of the statistical principle of regression to the mean. These are typically measurable or quantitative traits like heights or head size, and are believed to result from the individually small effects of many genes combined with environmental factors. Threshold traits do not appear until a certain threshold of liability is exceeded. Cleft lip and palate and pyloric stenosis are examples of threshold traits. Certain threshold traits have a predilection for one gender, indicating that males and females have a different liability threshold. The recurrence risk for threshold traits also is higher if the defect is severe, again indicating the presence of more abnormal genes or influences. For example, the recurrence risk after the birth of a child with bilateral cleft lip and palate is 8%, compared with only 4% after unilateral cleft lip without cleft palate. Complex disorders of adult life are traits in which many genes determine an individual's susceptibility to environmental factors, with disease resulting from the most unfavorable combination of both. This category includes common diseases, such as heart disease and hypertension (3).
IV. Teratogens or Environmental exposures:
Teratogens or environmental exposures are thought to cause congenital abnormalities in less than 1% of infants. This area is important because these exposures are potentially preventable. Such teratogens include medications, exposure to agents such as ionizing radiation, and by-products of some maternal metabolic disorders such as diabetes and phenylketonuria.
For details please review the chapter on: Drugs in Pregnancy and Lactation
Indications for Prenatal Diagnosis:
It is essential that at-risk couples be informed of all their reproductive options. Before attempting the prenatal diagnosis of any inherited disease, it is essential to establish or confirm the directed toward eliminating the possibility of misdiagnosis due to phenotypic, metabolic, or genetic heterogeneity. In the case of an enzymatic or molecular diagnosis, the precise defect must be demonstrated in the proband or affected relatives. If the proband is deceased, the heterozygosity of both parents (or of the mother for an X-linked disease) must be documented. In the case of dysmorphic syndrome, all manifestations of the disorder must be known and looked for at the time of evaluation.
The most common indications for prenatal diagnostic studies are: advanced maternal age; abnormal biochemical screening; previous child with a chromosomal disorder; balanced translocation carrier for a chromosomal disorder; previous child with an inherited metabolic disorder; heterozygous couples detected prospectively by screening programs; previous child with a disorder detectable by ultrasound.
Multifactorial disorders: previous child with neural tube defect; elevated maternal serum alpha-feto-protein (AFP) and previous child with developmental defect or malformation syndrome detectable by ultrasound.
Environmental defects: prenatal exposure to teratogenic drug or infectious agent.
Using a maternal age of 35 or older at the time of delivery or older at the time of delivery as an indication for invasive testing (amniocentesis or chorionic villus sampling), approximately 20% of Down syndrome cases will be detected. 80% of cases of Down syndrome will occur in the non-tested group. In an effort to increase the detection rate of Down syndrome, midtrimester maternal serum biochemical screening has been developed that detects approximately 60% of Down syndrome cases in the general population (4).
Biochemical Screening for Birth Defects:
Some of the greatest advances in genetics during the past two decades have occurred in the area of biochemical screening for birth defects in the fetus. A number of biochemical markers have now been studied for use in prenatal screening. These are:
Alpha-Feto-Protein (AFP):
AFP is an onco-fetal protein produced by the fetal liver and yolk sac and is the major serum protein in early fetal life. It initially enters the amniotic fluid via diffusion across the immature fetal skin and later through fetal urination. Non-pregnant women have levels of AFP that are barely detectable (1 u/L), whereas normal median levels at 16 to 18 weeks of gestation range from 18 to 40 u/L. Maternal serum AFP continues to increase until 28 to 32 weeks of gestation. In the fetus with a defect such as anencephaly or spina-bifida, AFP enters the amniotic fluid in increased amounts, leading to higher levels in the maternal serum as well. Levels of AFP are elevated in amniotic fluid and maternal serum only when such lesions are "open", i.e., when the neural tissue is exposed or covered by only a thin membrane. Virtually 100% of anencephaly lesions are open; approximately 20% spina-bifida cases are closed, as are about 82% of encephaloceles (5). Discussions of the sensitivity and specificity of AFP testing are generally restricted to open lesions, as it is assumed that closed lesions will not be detected.
In laboratories providing AFP screening, results are expressed as multiples of the median (MoM), which allows expression of measurements in a common way. The distribution of maternal serum AFP (MSAFP) is log-Gaussian, with median values of 1.0 for normal pregnancies, 3.8 for pregnancies with open spina-bifida, and 6.5 for pregnancies affected with anencephaly. The two most commonly used cutoffs for MSAFP are 2.0 and 2.5 MoM. At 2.0 MoM the false-positive rate ranges from 4% to 6%, and the detection rate for open spina-bifida ranges from 85% to 90%. With a higher cutoff of 2.5 MoM the false-positive rate decreases to 2% to 3% and the detection rate also decreases to 75% to 80%. Women with pre-gestational diabetes have MSAFP values that average 20% lower in the second trimester. When the MSAFP level is above the cutoff (either 2.0 or 2.5 MoM) the accuracy of dating is assessed. If dating is based on last menstrual period was used for the original calculation, and ultrasound should be performed for accurate dating. Amniocentesis may be indicated to identify the cause of elevated AFP.
Causes of an elevated MSAFP: multiple gestation; fetal demise; fetal-maternal hemorrhage; placental abnormalities; uterine abnormalities; maternal ovarian or hepatic tumors. Fetal Structural Defects: neural tube defects -- spina-bifida, anencephaly, encephaloceles; open ventral wall defects -- omphalocele, gastroschisis; congenital nephrosis; triploidy; bilateral renal agenesis; congenital skin disorders -- epidermolysis bullosa, aplasia cutis; autosomal recessive polycystic kidney disease; sacrococcygeal teratoma; cystic adenomatoid malformation of the lung.
Triple Marker Screening:
A number of other biochemical markers have now been studied for use in aneuploidy screening. Both unconjugated estriol (uE3) and human chorionic gonadotropin (hCG) add specificity and sensitivity to second trimester biochemical screening for Down syndrome. Triple marker screening with AFP, uE3, and hCG is now routinely offered in pregnancy. Most centers use a 1/270 risk of Down syndrome at midtrimester as the cutoff above which amniocentesis is offered; this risk is equivalent to that of a 35-year-old woman. As with AFP screening, maternal weight, race, diabetes, and the number of fetuses present are all considered in the risk calculation.
Fetal Tissue Sampling and DNA Methodology:
Some inherited diseases are expressed only in a specific tissue, and thus sampling of that tissue is necessary if a prenatal diagnosis is to be made. Fetal liver, fetal skin and fetal muscle have been obtained by ultrasonically guided biopsy (6). As progress is made in the elucidation of the specific molecular defect in an ever-increasing number of genetic diseases, prenatal diagnosis will be possible by DNA analysis. Because DNA is present in every nucleated cell, minimally invasive techniques such as amniocentesis or chorionic villus sampling may replace the more invasive procedures such as fetal blood sampling or fetal liver biopsy for the prenatal diagnosis of many diseases.
Ultrasound Evaluation of Chromosomal Abnormalities
Over the past decade and a half, the field of genetic sonography has emerged. Fifteen years ago amniocentesis was reserved for women of advanced maternal age, resulting in the potential for identifying less than quarter of the aneuploidy fetuses. Now the decision of whether a patient is at risk for fetal aneuploidy will be based on the combination of maternal age, multiple biochemical markers, and a complete ultrasound evaluation of the fetus. Perhaps using this combined method, we will progress from potential identification of 20% of fetuses with Down syndrome (i.e., those associated with a maternal age of >35 years) to be possible detection rate of 90% of fetuses with chromosomal abnormalities, while recommending amniocentesis to only 5% to 10% of pregnant women.
Ultrasound-Guided Diagnostic Obstetrical Procedures
Down syndrome is the most common chromosomal abnormality among live-borns and has also proved the most sonographically elusive syndrome of all the autosomal trisomic syndromes. It seems logical, however, that the sonographic examination of the fetus itself for the recognizable features of infants with Down syndrome would be even more sensitive and specific for the detection of affected fetuses than are maternal age and serum screening.
Sonographic Screening for Down Syndrome
Ultrasonographic Markers of Aneuploidy:
Second-trimester ultrasonographic examinations are often routinely performed to detect fetal anatomical abnormalities; the findings of a major anomaly or two or more minor structural malformations increases the likelihood of aneuploidy and warrants genetic counseling and consideration of amniocentesis (7). A number of fetal structural abnormalities and non-pathologic (or "soft") markers detectable on second-trimester ultrasonographic examination have also been associated with Down syndrome. Studies have shown that second-trimester ultrasonographic examination can identify 50 to 75% of fetuses with Down syndrome, at positive screening rates of 4 to 15%. However, test performance depends on the study population, choice of markers, gestational age, and expertise of the ultrasonographer. A common approach is to use likelihood ratios for ultrasonographic markers (calculated by dividing the sensitivity by the false positive rate) in order to calculate a woman's risk of having a fetus with Down syndrome; the risk based on maternal age or serum screening is multiplied by the likelihood ratio (8). In a prospective, population-based cohort study involving women with results of serum screening that indicated an increased risk of Down syndrome, the presence of structural anomalies or a thickened nuchal fold increased the risk of Down syndrome by a factor of nine, and absence of abnormalities reduced the risk by a third. When soft markers were detected in conjunction with a structural anomaly, the risk of Down syndrome was further increased, whereas the finding of soft markers alone (excluding the thick nuchal fold) did not substantively change the risk.
The significance of soft markers detected on routine ultrasonographic examination at 20 weeks' gestation in a patient who otherwise appears to be at low risk for giving birth to a child with trisomy 21 or trisomy 18 is uncertain, and a concern is that the presence of these markers may lead to invasive diagnostic procedures that are unnecessary. Conversely, a normal ultrasonographic examination may provide false reassurance to patients with a risk that is increased on the basis of serum markers or age. In the first trimester, non-visualization of the nasal bone on ultrasonographic examination has been associated with an increased risk of Down syndrome and other aneuploidies. When used with the first trimester combined screening in patients at increased risk because of maternal age (median age, 35 years), and estimated rate of detection of trisomy 21 was 93% at a positive screening rate of 5% (9). Assessment of the nasal bone has been incorporated into screening algorithms at some centers, but it should be performed only by trained ultrasonographers.
Ultrasonographic findings in Trisomy 21:
Structural anomalies: cardiac defects; cystic hygromas; duodenal atresia; ventriculomegaly.
Non-pathologic findings, or "soft markers": thick nuchal fold (>6 mm); hypoplastic or absent nasal bone; choroid plexus cyst; echogenic intracardiac focus; renal pyelectasis; short humerus; short femur; echogenic bowel; umbilical cord with two vessels; hypoplasia of middle phalanx of fifth finger; sandal-gap toe (separation of great toe)
n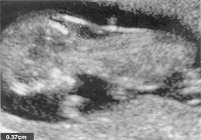
Increased nuchal translucency measurement of 3.7 mm at 12 weeks in a fetus with Down syndrome
Other first trimester ultrasonographic markers under investigation (e.g. ductus venosus on Doppler flow imaging, fetal tricuspid regurgitation, and abnormal fronto-maxillary and maxillary angles of the mandible) are not generally used in screening algorithms in routine practice (10). Prerequisites for the more widespread use of these measures include assessment in low-risk populations, the use of data on gestational age and norms that are specific to the patient's race or ethnic group, adoption of a standardized approach, adequate training, and implementation of quality-assessment programs.
Summary:
Ultrasound will continue to play a critical role in the development of these new approaches to fetal prenatal diagnosis and therapy. The availability of early first trimester prenatal diagnosis may provide opportunities for prenatal therapy of certain disorders by replacement of missing enzymes or cofactors, by somatic gene replacement, or by stem cell transportation. Safe and reliable access to the fetal circulation is opening up a new era in fetal prenatal diagnosis and fetal therapy.
References
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Invasive prenatal testing for aneuploidy. Obstet Gynecol 2007;110:1459-1467
- Nuaabaum RL, McInnes RR, Willard HF. Thompson & Thompson genetics in medicine. 7th ed. Philadelphia: Sunders/Elsevier, 2007
- The human genome. Science 2001;291:1145-1434
- Malone FD, D'Alton ME. First-trimester screening for Down syndrome. Obstet Gynecol 2003102:1066-1079
- Bahado-Singh RO, Oz UA, Mendilcioglu I et al. The mid-trimester genetic sonogram. Semin Perinatol 2005;29:209-214
- Simpson JL, Elias S. Genetics in obstetrics and gynecology. 3rd ed. Philadelphia, WB Saunders, 2003
- Alfirevic A, Sundberg K, Brigham S. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database Syst Rev 2003;3:CD003252
- Smith-Bindman R, Chu P, Goldberg JD. Second trimester prenatal ultrasound for the detection of pregnancies at increased risk for Down syndrome. Prenat Diagn 2007;27:535-544
- Rosen T, D'Alton ME, Platt LD et al. First-trimester ultrasound assessment of the nasal bone to screen for aneuploidy. Obstet Gynecol 2007;110:399-404
- Driscoll DA, Gross S. Prenatal screening for aneuploidy. N Engl J Med 2009;360:2556-2562
Published: 19 October 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


