The Bethesda System - An Overview
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
George Nicholas Papanicolaou is generally regarded as the father of cytopathology and in 1928 described cancer cells in the vaginal smears. The American Society of Cytology (ASC), organized in 1953 brought together pathologists, technologists, and clinicians dedicated to advancing the clinical application of cytology. In the 1950s the cytology literature began to proliferate. In 1961, Leopold Koss, an indefatigable worker in all phases of cytopathology published a landmark text, Diagnostic Cytology and its Histologic Bases, now in its fourth edition. By the 1980s, DNA determination by flow cytometry and cell image analysis began to enhance the diagnostic accuracy of cytopathology. The first Bethesda Conference met on December 12-13, 1988 in Maryland (USA) at the National Institutes of Health (NIH) to consider methods of reporting gynecologic cytology in meaningful diagnostic terminology. Between 1988 and 1991, The Bethesda System (TBS) nomenclature materialized on cytology reports in the United State, Europe, Asia and Latin America. Today both exfoliative and aspiration biopsy cytology have gained widespread acceptance.
Abbreviations:
AGUS - Atypical glandular cells of undetermined significance
APK - Atypical parakeratosis
ASCUS - Atypical squamous cells of undetermined significance
CIN - Cervical intraepithelial neoplasia
HPV - Human papillomavirus
SIL - Squamous intraepithelial lesion
HSIL - High-grade squamous intraepithelial lesion
LSIL - Low-grade squamous intraepithelial lesion
T-zone - Transformation zone
TBS - The Bethesda System
Format: The Bethesda System (TBS)
The TBS format and their appropriate usage are the main focus of this document (1). Specimen adequacy and the terminology and its clarification is discussed in detail:
- Specimen adequacy
- Within normal limits
- Benign cellular changes
- Epithelial cell abnormality
- Descriptive diagnoses
- Benign cellular changes
- Infection
- Reactive/reparative
- Epithelial cell abnormalities
- Squamous cells
- ASCUS
- LSIL (including HPV)
- HSIL
- Carcinoma
- Glandular cell
- Endometrial cells (benign, post-menopausal)
- AGUS - Atypical Endometrial Cells & Atypical Endocervical Cells
- Adenocarcinoma
- Endocervical
- Endometrial
- Extrauterine
- Other Malignant Neoplasms
- Undifferentiated small-cell carcinoma, melanoma, lymphoma, or sarcoma
- Hormonal Evaluation
1. Specimen Adequacy:
According to TBS four elements constitute the adequacy of the specimen and a specimen satisfactory for evaluation is one with all the following:
- Patient and specimen identification
- Pertinent clinical information
- Technical interpretability
- Cellular composition and sampling of the transformation zone
Adequacy relevant to the cellular content is defined as: squamous cells covering more than 10% of the slide surface and Transformation zone (T-zone) cells comprising at least two clusters of well-preserved Endocervical glandular and/or squamous metaplastic cells with each cluster composed of at least five cells (2). For atrophic smears, T-zone cells are not requisite because both metaplastic and endocervical cells may be indistinguishable from parabasal cells.
Analysis of adequacy factors begins with the clinician and the collection process: visualization of the cervix with appropriate sampling and proper slide preparation. The adequacy criteria are viewed as an initial attempt to develop a more standardized approach. The following explanation is illustrative (3):
- Satisfactory - "The smear has a high but not 100% likelihood of detecting a significant cervical epithelial abnormality. After at least three consecutive annual satisfactory normal smears, the risk of a missed abnormality is extremely low".
- Satisfactory but limited - "The smear gives useful information about the status of the cervix but, based on current knowledge, is somewhat less likely to detect a cervical epithelial abnormality or to accurately demonstrate the nature or severity of a lesion".
- Unsatisfactory - "The smear is unreliable for detection of cervical cancer or precursor lesions; a diagnosis of the presence or absence of an epithelial abnormality cannot be given".
General Categorization:
This element of The Bethesda System (TBS) was originally included to assist triage of reports by support staff. The categorization has three parts: Within Normal Limits, Benign Cellular Changes, and Epithelial Cell Abnormality. Descriptive diagnoses encompass inflammatory and reparative processes and hormonal evaluation, as well as lesions of squamous and glandular cell.
2. Descriptive Diagnoses:
Benign Cellular Changes - it provides for the diagnosis of organisms which may be identified cytologically with a high specificity. Recognition of the inherent limitations associated with identification of such pathogens through light microscopy and such findings are to be reported with qualifying phrases, such as: organisms morphologically consistent with Candida species, Actinomyces species, and herpes simplex alterations. Chlamydiae are excluded because of the low accuracy of this evaluation. Human papillomavirus (HPV) associated changes are described under "Epithelial Cell Abnormalities".
Reactive/Reparative Changes - this category encompasses benign cellular changes that are reactive in response to such factors as inflammation, radiation or an intrauterine device. It includes reparative changes, or "typical repair", which may be seen in squamous or glandular epithelium, usually in the presence of inflammation. Reactive cellular changes associated with atrophy and inflammation or atrophic vaginitis has been added (4). See the picture below:
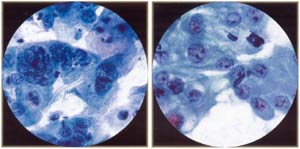 | |
Reactive endocervical cells. Note mild hyperchromasia, pleomorphism, multinucleation, and prominent nucleoli in some cells. Multinucleation should not be mistaken for viral effect (lacks characteristic ground glass chromatin of herpes.) |
Reactive/reparative endocervical cells. Reparative process is characterized by nuclear "atypia" (enlargement, prominent nucleoli, pleomorphism), but distinguished from cancer by orderly, cohesive groupings, pale chromatin, and lack of tumor diathesis. Mitotic figures, generally a worriesome feature in endocervical cells, can be seen in repair. |
3. Epithelial Cell Abnormalities (Squamous Cells):
This section includes ASCUS, LSIL, HSIL and squamous cell carcinoma.Atypical Squamous Cells of Undetermined Significance (ASCUS) - the diagnosis ASCUS is restricted to those cases in which the cellular changes exceed those attributable to benign, reactive processes but that fall short of a definitive diagnosis of SIL. The diagnosis should not be used for otherwise-defined benign inflammatory or premalignant processes. The diagnosis should be used for otherwise-defined benign inflammatory or premalignant processes. The diagnosis should be further qualified, when possible, to indicate whether a reactive process or SIL is favored. Hyperkeratosis, parakeratosis, and dyskeratosis are descriptors that have been used inconsistently in the past and are not included in TBS terminology (5). ASCUS is a designate for cells with mild nuclear abnormalities, which are not clearly distinguishable from either reactive/reparative cell or LSIL. These cells demonstrate alterations either in too small a degree or in too few cells to interpret as LSIL. ASCUS is a diagnosis of exclusion for cytopathologic findings that are not sufficiently clear-cut to permit a more specific diagnosis.
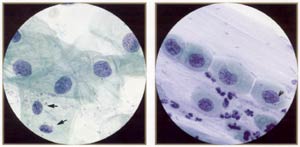 | |
Squamous atypia (ASCUS). Compare the atypical cells with the normal intennediate cells in the field (arrows). The atypical nuclei are enlarged (-2 to 3 or more times the size of the normal intennediate nucleus) and usually slightly hyperchromatic. The nuclear membranes are smooth or minimally irregular, and the chromatin is fine and evenly distributed, without granularity. Note that the cytoplasm of the atypical cells tends to be thin and delicate, like normal mature cells. |
Atypical squamous metaplasia (ASM) (ASCUS). ASM is a difficult diagnosis: the nuclei are very subtly abnormal, less abnormal than even mild (immature) metaplastic dysplasia. As in squamous atypia, the nuclei are enlarged into the range of 70-120 μm². The nuclear membranes are smooth or only slightly irregular, and the chromatin is fine and evenly distributed, without granularity. |
Squamous Intraepithelial Lesion (SIL) - the "SIL" category includes HPV changes. Cells must exhibit nuclear enlargement as well as a defined, perinuclear clear area demarcated by a dense cytoplasmic ring (koilocytes); there may be nuclear smudging and binucleation. By contrast, cells with no nuclear enlargement but showing perinuclear halos, keratinization, and/or multinucleation are to be interpreted as benign.
LOW-GRADE SQUAMOUS INTRAEPITHELIAL LESION (LSIL) - is synonymous with CIN I or mild dysplasia by with it may be qualified, either without or with HPV changes. Interpretation is based on cells with nuclear enlargement to at least three times that of the normal intermediate cell and an increased nuclear/cytoplasmic ratio. The superficial and intermediate cells, in sheets or isolated are generally involved. The nuclear contour may be slightly irregular with hyperchromasia and/or smudged or granular but evenly distributed chromatin. When rare cells with these abnormalities are present, it can be reported as; a few cells suggestive of LSIL.
HIGH-GRADE SQUAMOUS INTRAEPITHELIAL LESION (HSIL) - is synonymous with both CIN II or moderate dysplasia as well as CIN II as well as CIN III or severe dysplasia/CIS, may be so qualified. Alterations are more pronounced in cells from HSIL than from LSIL. Generally the former are more abundant, smaller in size, and more immature. Syncytia and isolated cells are more common than sheets. Nuclear changes include an increased nuclear/cytoplasmic ratio, greater irregularity of contour, and coarsened and/or clumped chromatin. Traditionally, cells from CIN III are smaller than those from CIN II and are either isolated with markedly enlarged nuclei and/or grouped in syncytia. Although HSIL subtyping is not required by TBS, distinction whenever possible is recommended.
SQUAMOUS CELL CARCINOMA - it is subdivided as keratinizing or nonkeratinizing in the Bethesda scheme (6). Criteria for both include (a) isolated and syncytia-forming malignant cells with variation in size and shape, (b) nuclei with irregularly distributed, coarse chromatin, ( c) macronucleolie and (d) a tumor diathesis. Features more common to keratinizing carcinoma are isolated, plemorphic cells with dense, orangeophilic cytoplasm and irregularly contoured nuclei, while those more common to nonkeratinizing tumors are syncytia and macronucleoli. Specimens with syncytia but no tumor diathesis may be misinterpreted as HSIL (CIN III). Undifferentiated cell-types, including the so-called small-cell carcinoma, are classified under "Other malignant neoplasms".
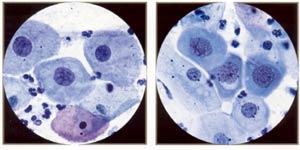 | |
Low-grade squamous inttaepithelial lesion: mild dysplasia, mature metaplastic type (aka large cell, nonkeratinizing dysplasia). Note resemblance to normal intermediate cells, although the cytoplasm is slightly thick and dense. However, the nuclei are "big and dark," ranging from 120 to 210 μm² (compared with 35 μm² for the normal intermediate nucleus). |
High-grade squamous intraepithelial lesion: moderate dysplasia, immature metaplastic (or simply metaplastic) type. Note resemblance to ordinary immature squamous metaplasia (parabasal-sized cells) except that the nuclei are "big and dark" (120 to 210 μm² compared wiili 50 μm² of normal metaplastic nucleus). |
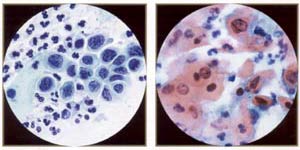 | |
High-grade squamous inttaepitheliallesion: severe dysplasia, (very immature) metaplastic type. This is represented by small cells with high nuclear/cytoplasmic ratios and irregular, "raisinoid" nuclei. Note thin rim of dense cytoplasm (compare with carcinoma in situ). |
High-grade squamous intraepithelial lesion: keratinizing dysplasia, aka pleomorphic dysplasia - a good descriptive term for higher grade keratinizing lesions. |
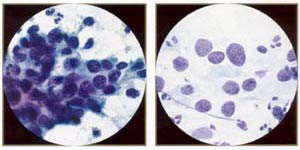 | |
High-grade squamous inttaepithelial lesion: carcinoma in situ. The most characteristic feature of CIS is the presence of three-dimensional syncytial aggregates of cells with scant, delicate cytoplasm, loss of nuclear polarity, and abnormal nuclei. Syncytial aggregates of CIS are important forms of "hyperchromatic crowded groups." |
High-grade squamous intraepithelial lesion: carcinoma in situ. Because of the delicate nature of the undifferentiated cytoplasm, numerous atypical naked nuclei may be present. Note, however, that naked nuclei are not diagnostic in themselves of CIS. |
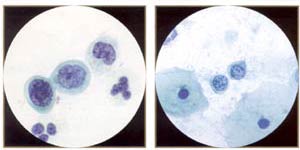 | |
Small, bland, severely dysplastic cells indicate very immature metaplastic dysplasia. These small cells (compare with neutrophil), indicative of severe dysplasia (CIN III, high-grade SIL), can be difficult to spot and difficult to distinguish from benign immature metaplasia or even histiocytes. Note irregular, "raisinoid" nuclei. (Oil) |
Small, bland, malignant cells. Despite the fairly bland chromatin and smooth nuclear membranes, these are fully malignant cells from a biopsy proven invasive squamous cell carcinoma of identical morphology. They would be easy to overlook in screening (a possible example of litigation cells). |
4. Glandular Cells:
This section includes benign endometrial cells, atypical glandular cells of undermined significance (AGUS) derived from either endocervical or endometrial cells, and adenocarcinomas (originating in the endocervix, endometrium, or extrauterine area).Benign Endometrial Cells - benign out-of-cycle endometrial cells are excluded from TBS, while those from postmenopausal women not on exogenous hormones must be explained. Endometrial cells may be present when dislodged from the lower uterine segment by endocervical brushing. They also may be seen because of benign polyps or hyperplasia. Occasionally, however, they are shed secondary to hormone producing neoplasms of the tubes or ovaries.
Atypical Glandular Cells of Undetermined Significance (AGUS) - the definition of AGUS according to TBS is cells showing either endometrial or endocervical differentiation displaying nuclear atypia that exceeds obvious reactive or reparative changes but lacks unequivocal features of invasive adenocarcinoma. The diagnosis should be qualified if possible to indicate whether the cells are thought to be of endocervical or endometrial origin. Atypical endocervical cells may be subclassified further according to whether a reactive or neoplastic process is favored. Criteria for separating atypical endometrial cells are not well defined and therefore this category is not further subdivided (7).
Atypical Endometrial Cells - cytoplasmic borders are ill defined, and there may be vacuoles. Nuclear changes include enlargement, hyperchromasia, and micronucleoli.
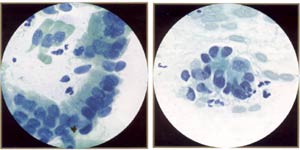 | |
AGUS (atypical endocervical cells). These endocervical cells are somewhat hyperchromatic, crowded, and stratified, features that may suggest endocervical glandular neoplasia. However, these cells represent robal metaplasia. Note round, rather than elongated, nuclei and bland, rather than coarse, chromatin. Also note ciliated cells. |
AGUS (atypical endocervical cells). These endocervical cells are forming a microacinar sttucture, a feature that may suggest endocervical glandular neoplasia. However, these cells also represent tubal metaplasia, with bland, round nuclei. |
5. Adenocarcinoma:
Atypical Glandular Cells - Comparative Cytomorphology
Source: Critical Issues in Cytopathology
| Distinctive Features |
Reactive | LSIL | HSIL | Adenocarcinoma |
| Structure | Monolayered | Crowded | Piled | Multilayered |
| Cytoplasm | Defined | +/- | +/- | +/- |
| Nuclei: 1. Contour 2. Chromatin |
Smooth Fine |
Smooth Smudgy |
Irregular Coarse |
Irregular Clumped |
| Nucleoli | Macro | +/- | Micro/Macro |
6. Other Malignant Neoplasms:
The cytopathologist should provide as specific a diagnosis as possible to guide the clinician in further evaluation of the patient. This also includes small-cell carcinoma, now considered a neuroendocrine tumor according to the 1994 WHO classification rather than variant of the squamous cell carcinoma.
7. Hormonal Evaluation:
The inclusion of hormonal evaluation in TBS was subjected to considerable debate prior to its insertion into the classification. This descriptive diagnosis can be applied only to vaginal smears (8). By TBS menu there are three possible statements: the specimen is compatible with age and history; or the specimen is incompatible with age and history; or hormonal evaluation is not possible due to...
Summary:
The Bethesda System terminology increasingly is being applied to reports throughout the United States. Internationally TBS slowly is being accepted. An obstacle, however, is the inconsistent nomenclature between cytologic and tissue studies. Uniform reporting system of cytologic classification if accepted and used worldwide, would have significant benefits, not only to unify interpretation, but also in the exchange of information for research and epidemiological study of the disease.
References:
- Wright TC, Massad LS, Dunton CJ et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol 2007;197:346-355
- Cox JT, Schiffman M, Solomon D, ASCUS-LSIL Triage Study (ALTS) Group. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol 2003;188:1406-1412
- ASCUS-LSIL, Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undermined significance. Am J Obstet Gynecol 2003;188:1383-1392
- Duggan MA. Cytologic and histologic diagnosis and significance of controversial squamous lesions of the uterine cervix. Mod Pathol 2000;13:252-260
- Crum CP. Symposium part 1: should the Bethesda system terminology be used in diagnostic surgical pathology? Int J Gynecol Path 2002;22:5-12
- Uterine Cervical Cytology. In Surgical Pathology; 9th edition. Editors: Rosai and Ackerman 2004. Publisher Mosby
- International Collaboration of epidemiological Studies of Cervical Cancer, Appleby P, Bernal V et al. Cervical cancer and hormonal contraceptives: collaborative reanalysis of individual data for 16,573 women with cervical cancer and 35,509 women without cervical cancer from 24 epidemiological studies. Lancet 2007;370-1609-21
- Moscicki A, Ma Y, Wibblesman C et al. Risks for cervical intraepithelial neoplasia 3 among adolescents and young women with abnormal cytology. Obstet Gynecol 2008;112:1335-1342
Published: 2 December 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


