Cervical Cancer Prevention: Managing Low-Grade Cervical Neoplasia
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Cervical cancer is the most common cancer in women, and caused by the human papillomavirus (HPV). Most sexually active women will acquire HPV in their lifetime. Although infection clears in most cases, it does persist in some women. Long-term persistence of HPV -- particularly with high-risk types -- has been established as a necessary cause of precancerous lesions. Cervical screening programs have been very successful in the United States, Europe, and other regions, are able to achieve broad and sustained coverage. Cervical cancer prevention can now be made even better. Substantial modifications of practice are forthcoming, motivated by improved understanding of HPV natural history and cervical carcinogenesis. Based on the central role of persistent cervical infections by carcinogenic HPV genotypes in cervical carcinogenesis, HPV DNA testing has been introduced into cervical cancer screening. In 1999, the U.S. Food and Drug Administration (FDA) approved the first carcinogenic or high-risk HPV DNA test for triage of equivocal cervical cytology and in 2003 for cotesting of women 30 and older (Hybrid Capture 2; Qiagen Corporation, Gaithersburg, MD). The American Cancer Society (ACS) in 2002 and a workshop co-sponsored by the National Institutes of Health (NIH)-National Cancer Institute (NCI), the American Society of Colposcopy and Cervical Pathology (ASCCP), and the ACS in 2004 published guidelines for adjunctive HPV testing with cervical cytology (cotesting) for cervical cancer screening of women aged 30 years and older in the United States. Recent evidence has shown that the risk of malignant and premalignant cervical disease and HPV infections varies significantly with age. The essentials of cervical cancer prevention have not changed much in the past 50 years. Technology has advanced, but without altering the basic strategy or performance. Cervical cancer screening programs have been very successful because cervical cancer usually develops slowly over decades. Repetitive rounds of screening catch precancerous lesions as they grow, while they can be easily treated.
The purpose of this document is to discuss the relationship of HPV detection and abnormal cytology with histologic diagnoses of cervical precancer and cancer. The focus of this discussion is on management of atypical squamous cells of undermined significance (ASCUS) and low-grade squamous intraepithelial lesions (LSIL). This series on Cervical Cancer Prevention, will also serve as a baseline for understanding the future effects of HPV vaccination on the cervical cancer screening results. The future of cervical cancer prevention will likely be more effective, because it will include: prophylactic vaccination of adolescents against carcinogenic HPV infections; an increased role for HPV testing; improvements in colposcopy to increase sensitivity; and reduction in the number of lifetime screens needed for prevention.
Abbreviations Used:
Adenocarcinoma in situ -- AIS
American Cancer Society -- ACS
American College of Obstetricians and Gynecologists -- ACOG
American Society for Colposcopy and Cervical Pathology - ASCCP
Atypical glandular cells -- AGC
Atypical squamous cells -- ASC
Atypical squamous cells of undermined significance -- ASCUS
Atypical squamous cells, cannot exclude HSIL -- ASC-H
Cervical intraepithelial neoplasia (mild or grade 1) -- CIN1
Cervical intraepithelial neoplasia, (moderate or grade 2) -- CIN2
Cervical intraepithelial neoplasia, (severe or grade 3) -- CIN3
Fluorescence in situ hybridization -- FISH
Large Loop Excision of Transformation Zone -- LLETZ
Low-grade squamous intraepithelial lesion -- LSIL
High-grade squamous intraepithelial lesion -- HSIL
Human papillomavirus -- HPV
Background
In 2007, over 13,000 women were diagnosed with cervical cancer in the United States. Use of Papanicolaou (Pap) smear has been credited with helping to prevent cervical cancer mortality and reduce cervical cancer rates by more than 70% (1). However, in the United States, low-grade squamous intraepithelial lesions (LSIL), the initial step to cervical dysplasia, is annually diagnosed in approximately one million women. Infection with high-risk strain of HPV is considered to be the most important factor in the initiation of cervical carcinogenesis. Consequently, HPV screening has become widespread and is an important part of risk stratification and clinical management guidelines. The key to developing effective guidelines for the management of cervical abnormalities is to distinguish true cervical cancer precursor from benign cervical abnormalities with little premalignant potential. Both LSIL and CIN1 reflect the cytologic and pathologic effects of infection with HPV. Most of these lesions will not progress to cancer. However, as many as 28% of women with cytologic LSIL harbor CIN 2 or CIN 3, approximately two thirds of which is identified by colposcopy (2). From a clinical perspective, it is important to distinguish which intraepithelial neoplastic lesions will progress to invasive cancer if left untreated. However, the diagnostic categories currently available have only modest predictive value, and that value decreases as the lesions become less severe. The likelihood of progression to cancer is higher and the time to progression is shorter as the grade of dysplasia increases.
HPV and Cervical Cytology
A high prevalence of HPV, especially among young women, does not result in cervical disease in most of the cases. Steadily decreasing, high-risk HPV prevalence across increasing age groups is consistent with other studies in the United States and other developed countries where cervical screening programs are common (1). It is likely that the immune system clears the HPV virus over time and that the development of cervical neoplasia in some individuals may pertain to failure of the immune system or to other genetic or environmental factors specific to those individuals. The findings that rates of cervical cancer differ among races may, as the authors observed (1), relate to differences in the natural history of the infection among races or to differences in diagnosis and cure. At any rate, the HPV virus appears to be involved in the development of cervical neoplasia, and hopefully, the immunization program now in place for young women will reduce this risk.
According to the American Cancer Society (ACS), there were an estimated 9,710 new cases of cervical cancer and 3,700 deaths from cancer of the cervix in the United States in 2006 (2). The primary cause of cervical cancer is HPV, a sexually transmitted infection that varies in prevalence depending on age and number of sexual partners. Prevalence is highest among sexually active young women who are being exposed for the first time, and tapers off after age 50 years. Women under age 24 years are generally able to clear an HPV without sequelae, but this becomes less likely with increasing age of detection. The cervical smear is a very effective screening test for cervical cancer and precancerous lesions that has been used since the mid-20th century, well before the link to HPV was discovered. Until recently, it was the only screening test available for cervical dysplasia. In February 2003, the U. S. Food and Drug Administration approved and HPV DNA test that could be used with cervical cytology to detect women at risk for cervical cancer and precancerous lesions. More than 100 subtypes of HPV currently have been identified, and over 40 of these subtypes can be found in the cervix or vagina. The HPV DNA testing technique detects multiple subtypes that are found in cervical cancer. Based on the available evidence, the National Cancer Institute, the American Society for Colposcopy and Cervical Pathology, and the ACS determined that women aged 30 and over may be screened using both cervical screening and the HPV DNA test concurrently. Women, for whom both tests are found to be negative, have a risk of cervical intraepithelial neoplasia 2 or 3 that is approximately 1 in 1,000 (3). It is therefore recommended that they be re-screened no more frequently than every 3 years. Women with negative cytology but positive high-risk HPV should have both tests repeated in 6-12 months. Those with abnormal cytology or persistent high-risk HPV should undergo colposcopy. For women under age 30 years, the prevalence of HPV is very high although the risk of cervical cancer is very low, thus screening for HPV in all women under age 30 years would lead to unnecessary additional testing such as colposcopy or repeat cervical screening, with few findings of significance. The current recommendation is to perform cervical screening with HPV reflex testing for HPV only with a cervical smear that shows atypical squamous cells of undermined significance (ASCUS) (4).
In a well-organized cervical cancer screening program, the HPV test is more sensitive than conventional cytology for detecting CIN 3 lesions and cancer (5). In this large, randomized trial from Finland, the HPV test functioned effectively as the primary screen for cervical cancer and was more sensitive than conventional cytology for detecting cervical intraepithelial neoplasia grade 3 (CIN 3) or higher. The greatest strengths of this study are the 1:1 randomization of just over 58,000 women and the ability to link study participants to outcomes, over a 5-year period, using the comprehensive Finnish population database and cancer registry. One concern that clinicians may have is whether the findings are applicable to a U.S. population that is now rarely screened using conventional cytology (liquid-based cytology is the norm). That concern should be allayed by a large meta-analysis that found no difference in the sensitivity of liquid-based cytology versus conventional Pap testing (6). Despite the large size, the study had limited statistical power to show the impact of the two screening modalities on the rate of cervical cancer, primarily because that rate is so low in the population screened. To determine that impact, the screening options need to be repeated for another round, with follow-up extended to 10 years.
Clinical Application of HPV Testing
Cotesting is the standard: US guidelines from the ACS (2002) and ACOG (2003, 2009) offer clinicians the option of screening women 30 years and older using both cytology and HPV testing -- an approach known as "cotesting". However, even though about 90% of the women who have a negative response to both tests can safely forgo further screening for at least 3 years, many clinicians screen them more frequently with cotesting, decreasing the cost-effectiveness of this options (7). We recommend: that you follow current US guidelines and screen women 30 years and older with both the Pap and HPV tests and extend the screening interval to 3 years for women who have a negative results on both tests. Numerous studies support the overwhelming conclusion that HPV testing in primary cervical cancer screening significantly increases detection of CIN 3 or higher and should reduce the woman's subsequent risk of developing cervical cancer.
There are two main criteria for deciding whether HPV-based triage is clinically useful. First, does the triage test (HPV testing) identify a significant proportion of women who need or do not need referral to expensive colposcopy and biopsy procedures that justify the cost of the triage test? That is, if HPV testing is almost always negative or positive, there is little value in using it. As an example of the latter, the HPV testing arm for women referred into the ASCUS-LSIL Triage Study (ALTS) with LSIL cytology was terminated before completion because most tested HPV positive (8). The second criterion is, whether HPV-negative women can be safely followed rather than sent to colposcopy. By these criteria, HPV triage of women 45 years and older or 50 and older with LSIL may be rational because of the lower percentage of those HPV positive (less than 80%) are at the very low risk of CIN3 and cancer among the HPV-negative women. This is observed in a large clinical trial in Europe (9). The discrepancy between the observations and ALTS is that ALTS enrolled predominately younger women (median age 24 years) (8). HPV testing may be useful for triage for colposcopic referral for LSIL cytology in older women but not for ASC-H cytology at any age (11).
HPV test results for women with AGC cytology may not be useful for triage, but may guide clinicians to which anatomical site, cervix or endometrium, a problem may exist: women with AGC cytology who test HPV positive have exceptional high risks of cervical precancer and cancer while those who test HPV negative have a high risk of endometrial cancer (10).
HPV testing will become increasingly important to distinguish women at high and low risks of cervical precancer and cancer among HPV-vaccinated women with equivocal and mildly abnormal cytology. In HPV16/18-vaccinated population, this study (11) observed fewer interpretations of HSIL cytology and less disease associated with any abnormal cytologic result because HPV16 preferentially causes HSIL cytology and HPV 16 and 18 are two most carcinogenic HPV genotypes. Partial protection by HPV vaccines against the next most carcinogenic HPV genotypes, especially HPV31 and HPV45, may further reduce the occurrence of HSIL cytology. Thus, there will be a profound shift toward equivocal cytology in HPV-vaccinated populations.
Molecular Markers of Cervical Dysplasia
Research developments in the molecular pathogenesis of cervical cancer have focused on the development of molecular markers for use in cervical cancer screening. Specifically, amplification of chromosome arm 3q has been demonstrated in cervical carcinomas and has also been observed in other tumors (12). Comparative genomic hybridization (CGH) using archival samples demonstrated that DNA amplification on chromosome 3 (region 3q24 to 3q28) was associated with cervical carcinogenesis. It was subsequently determined that a probe targeting sequences for the RNA component of the human telomerase gene (TERC), located on chromosome region 3q26, could be used as a screening test for 3q amplification and might help assess the progression of an individual lesion (13). It is hypothesized that because a high percentage of dysplastic lesions have extra copies of the TERC gene, TERC/3q may have a causative, yet unproven role, in dysplasia via telomerase-based cell immortalization. Additionally, other genes localized to the 3q26 region, such as PIK3CA, which encodes a catalytic subunit of phosphatidylinositol 3-kinase and is associated with a number of cancer-related functions including apoptosis and cell growth, have the potential to be involved as cervical cancer oncogenes.
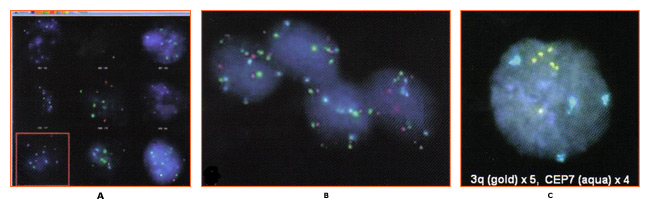
Research examining the association of 3q gain with cervical carcinogenesis shifted to investigation of the possibility that 3q gain can predict cervical disease progression. This study (14) demonstrated that while 3q gain predicted progression in 100% of cases (CIN1/2 progressing to CIN3); the absence of 3q gain was also able to predict 70% of regressor. Other research demonstrated that biomarker such as 3q (3q26) are temporally associated with HPV integration into the host genome (12). Therefore, a test that determines 3q status should be of use in triaging LSIL patients. Until recently, routine testing for 3q gain was not feasible because assessment required the analysis of a large number (800) of stained, squamous cell nuclei which is impractical by conventional microscopy. However, the oncoFISH® cervical test is a qualitative fluorescence in situ hybridization (FISH) test for determining the acquisition of specific chromosomal aneuploidies (within the 3q26 region) in cytological specimens.
oncoFISH is performed on cervico-vaginal cytology specimens, identical to those used for Pap and HPV testing. The test assesses amplification of the 3q26 region by use of two FISH probes, one for the 3q26 locus and a control probe. Enumeration and comparison of the 3q26 and control probes, in conjunction with the nuclear morphology, result in a 3q copy number for each of the nuclei analyzed. Although several factors contribute to the progression of a precancerous lesion to malignancy, 3q26 gain is a marker that the medical professional can use, in conjunction with other tests, to aid LSIL patient management.
nThe identification of a relationship between gain in the chromosome 3q26 region and development of cervical dysplasia has resulted in a number of studies confirming the association and suggesting the possible clinical utility of that observation. However, applying FISH-based analysis of liquid cytology specimens for 3q gain in a clinical setting is challenging. Screening all the cells present in the specimen for their FISH signals is impractical using traditional microscopy analysis. The automated nature of the assay allows for a comprehensive approach to specimen analysis that is not possible manually. Analysis of LSIL samples identifies up to 20% of specimens that show a level of 3q gain above that observed in normal specimens (29). A fully automated method for determination of 3q gain in liquid cytology may be the assay necessary to implement routine testing (29). Additional studies to validate the utility of this technology are needed. Further studies using this test based on the presence of cells with more than five 3q26 FISH signals, will allow better assessment of its clinical performance and its potential utility, in conjunction with existing tests, for improved patient management.
Detection of 3q gain in an archival LSIL specimen
n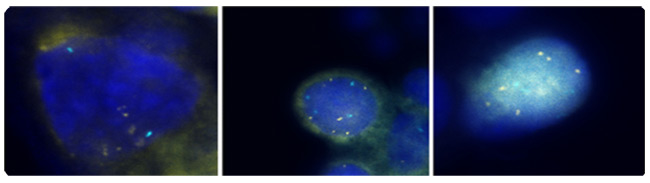
Figure 2: 3q fluorescence in situ hybridization (FISH) signals are colored gold and control centromeric 7 FISH signals are colored aqua. All 3 cells exhibit more than 4 3q (gold) FISH signals. Photographs courtesy of Ikonisys Inc.
Clinical Application of oncoFISH Cervical Test
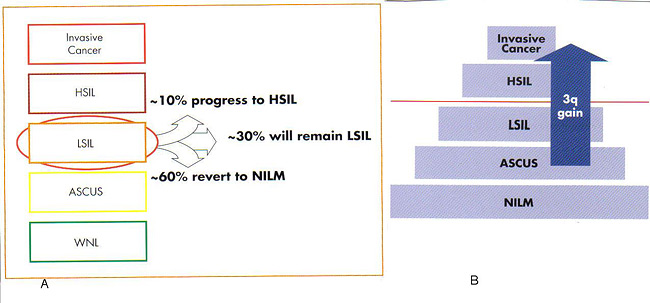
oncoFISH cervical provides physicians with additional information that can assist them in making more informed clinical management decisions. Which LSIL will progress clinically to a higher grade? Which will regress to normal? Approximately 10% of patients diagnosed with LSIL will progress to HSIL, 60% will regress to normal, and 30% will remain LSIL (15). oncoFISH cervical helps physicians assess which LSIL patients may regress and suggest LSIL cases at higher risk for disease progression. Among many chromosomal changes observed in cervical cancer, the most consistent abnormality is detected in chromosome arm 3q. Studies have shown that at least 90% of invasive cervical cancer cases have a gain in the 3q arm (14). Additional research has demonstrated a correlation between the gain in the 3q26 copy number as the severity and stage of cervical disease progress (13)(14). Using this technology to look at the progression of individual patients, it has been shown that the sensitivity of the 3q26 loci for predicting progression from CIN1/CIN2 to CIN3 was 100% and the specificity, i.e. the prediction of regression was 70% (14). oncoFISH cervical provides information for the clinical assessment of dysplasia prior to colposcopy.
nCervical Transformation Zone (TZ)
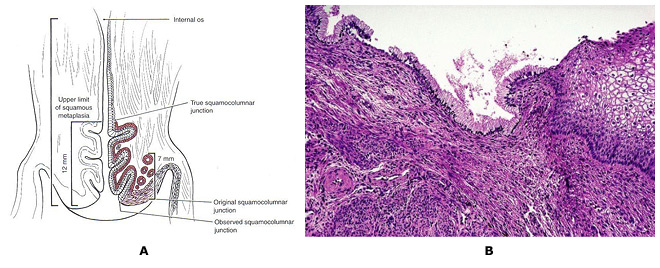
Carcinogenic HPV infections are particularly prone to cause cancer at transformation zones (TZs) where two kinds of epithelium meet, e.g., the cervix, anus and oropharynx (16). The transformation zone (TZ) is defined as that area lying between the original squamocolumnar junction and the colposcopic new squamocolumnar junction. The original linear junction between squamous and columnar epithelium is replaced by a zone of squamous metaplasia at varying degrees of maturation. At the upper or cephalad margin of this zone is a sharp demarcation between epithelium, which appears morphologically squamous using the magnified illumination of a colposcope, and erythematous, villous epithelium, which appears colposcopically columnar. This colposcopic junction is called the new squamocolumnar junction. The initial clinical assessment for most women is in the post-pubertal years. Mature squamous metaplastic epithelium has often replaced the distal or caudad limit of the columnar epithelium. The original squamocolumnar junction is most often seen clinically as a squamocolumnar junction separating original squamous and metaplastic epithelia. As the transformation zone matures, this junction becomes impossible to delineate. Only the presence of nabothian follicles and gland openings hints at the original columnar origin of mature squamous metaplasia. Cervical neoplasia almost invariably originates within the TZ. Understanding squamous metaplasia is the key to understanding the concepts of the cervical TZ and cervical carcinogenesis. If the new squamocolumnar junction is seen in its entirety in the absence of premalignant disease, the incidence of squamous disease above or cephalad to the new squamocolumnar junction is virtually nil. Thus, if the new squamocolumnar junction is seen in its entirety, the colposcopic examination of the cervix is described as satisfactory. If the new squamocolumnar junction is not seen its entirety, the colposcopic examination is described as unsatisfactory. The transformation zone further defines the distal limit of high-grade glandular intraepithelial neoplasia, the precursor lesion to invasive adenocarcinoma of the cervix.
nAlthough HPV infections are very common at genital sites such as the vagina, penis and vulva, rates of HPV-induced cancer are much lower owing to the lack of TZs. Squamous metaplasia continues throughout a woman's life, and eventually, the TZ is located inside the internal os. Hence, it is critical to sample the TZ in screening, but a sampling centered at the os will not always collect the optimal cells. Atrophy can reduce cell yields as well. There is hope that HPV testing including endocervical cells might reduce false-negative screening compared with cytology. However, whichever screening method is used, a diagnostic problem remains; even when properly performed, colposcopists cannot easily diagnose lesions at the poorly visible TZ among postmenopausal women.
n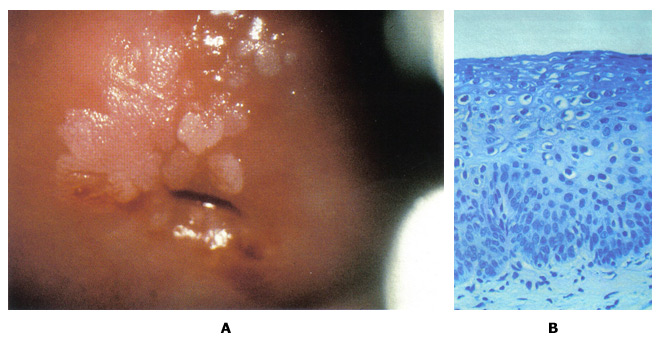
Figure 5: A. Colposcopic photograph of the cervix after the application of the acetic acid demonstrating numerous acetowhite lesions consistent with a low-grade squamous intraepithelial lesion (LSIL). B. Histologic findings show loss of polarity in the lower third of the epithelium and numerous koilocytes, consistent with cervical intraepithelial neoplasia, grade 1, with HPV features.
Cytology and Histology Findings and Interpretations
In the Bethesda 2001 guidelines, ASC is subcategorized into atypical squamous cells of undetermined significant (ASCUS) and atypical squamous cells, cannot exclude HSIL (ASC-H). The difference in the management guidelines for these two cytology findings relates to their inherent risk for CIN2,3. ASCUS is the most common cervical cytology abnormality, accounting for 4.4% of all Pap test results. Although the risk of cancer for any individual patient is very low (0.1-.02%) (19), and the risk of CIN2,3+ also is low (6.4-11.9%) (22), because there are so many people with this cytology abnormality, it is the presenting cytology result for approximately one half of the women with CIN2,3+. The first step in evaluation of women with ASCUS is to triage those who are at higher risk to more intensive evaluation (colposcopy) and directing the test to more routine follow-up. Triage testing may be performed by a single test for high-risk (oncogenic) types of HPV or by repeat cytology screening at 6 months and 12 months. When the index cytology test specimen is obtained by liquid-based cytology or when an HPV specimen is co-collected, "reflex" HPV testing is the preferred approach (17). Data from ALTS demonstrated that two repeat cytology examinations at 6 months and 12 months at an ASCUS threshold detected 88% of the CIN2,3+ while referring 63.6% of the women to colposcopy. HPV testing alone detected 92.2% of the CIN2,3+ while referring 55% of the women to colposcopy.
Management of Normal Cervical Cytology with HPV Test Positive:
The best management approach for HPV-positive, cytology-negative women 30 years and older is to repeat cytology and HPV testing at 12 months (17). Women whose HPV result is still positive on repeat testing 12 months later or whose cytology result is ASC or greater should undergo colposcopy, whereas women whose results are negative on both tests can defer screening for 3 years. Incorporating HPV testing into routine screening should be reserved for women aged 30 years and older (22). In screening studies from North America and Europe, the sensitivity using a combination of HPV testing and cytology is significantly higher than that of either test alone with negative predictive values of 99-100% (22). Women who receive negative results from both initial cytology and HPV testing have a less than 1 in 1,000 risk of having CIN2 or greater, and prospective follow-up studies in both Europe and the United States have shown that the risk of developing CIN3 over a 10-year period is less than 2% (22)(23). Modeling studies demonstrate that in women 30 years and older, screening at 3-year intervals using a combination of cytology and HPV testing provides benefits equivalent or greater than those provided by annual screening with conventional cytology. Even in women 30 years and older, most HPV-positive women become HPV-negative during follow-up (60% in a prospective study) (22). In a well-screened population, the risk of CIN2+ in HPV-positive, cytology-negative women range from 2.4% to 5.1% (23).
Management of Cervical Cytology ASCUS and LSIL in Adolescents (before age 21 years):
Invasive cervical cancer is very rare in adolescent women before age 21 years. The National Cancer Institute's SEER program reported that from 1995 to 1999 the incidence rate of invasive cervical cancer was 0 per 100,000 per year for women aged 10-19 years and 1.7 per 100,000 per year for women aged 20-24 years (1). Because the rate of HPV DNA infection or positivity is highest in the adolescent population, and because most HPV infections in adolescents with or without abnormal cytology will resolve without treatment, the current guidelines do not recommend the use of HPV testing in this population under any circumstance, including triage of ASCUS and follow-up of LSIL (17)(18). The ASCCP 2006 consensus guidelines for the management of the ASCUS and LSIL in adolescents (20 years and younger) recommend repeat cytology at 12-month intervals for a period of 2 years. If ASCUS or LSIL persist for a period of 2 years, clinicians are advised to perform colposcopy (17). This recommendation represents a significant change from previous guidelines and is based on natural history studies of ASCUS and LSIL that demonstrate a high rate of resolution of the disease within 2-3 years (18). The very similar clinical outcome of young women with ASCUS and LSIL has prompted ASCCP to recommend a similar management approach for both of these diagnoses. Assuming that CIN2 or greater has been ruled out by colposcopy, prospective studies of an adult population with CIN1 demonstrate that the risk of CIN2 or greater developing throughout a 2-year period is approximately 10% (19). In adolescent population, the rate of progression is much lower, estimated at 3%, whereas the rate of resolution of CIN1 is extremely high (greater than 90%) (18)(19). Therefore, initial management of CIN1 without therapy is mandatory.
Special considerations: minors undergoing a colposcopic examination may find it helpful to have parental involvement for the procedure. However, colposcopic examinations are considered evaluation for sexually transmitted diseases (STDs), and minors are generally are allowed to consent for diagnosis for diagnosis and treatment of STDs. For that reason, parental consent although preferred, should not be required. If parental consent is not obtained, consent for the examination should be obtained from minor and indicated in the medical record. Any health care provider who delivers such care should be fully informed of their state laws and established local standard of care.
Management of Cervical Cytology ASCUS and LSIL in Pregnancy:
Pregnancy in adolescents does not alter screening and management of abnormal cytology. Endocervical curettage and excisional procedures should never be performed during pregnancy unless invasive cancer is highly suspected (20). Screening for pregnancy, therefore, should be performed before evaluation and management of abnormal cervical cytology in adolescents. In pregnancy, the only diagnosis that may alter management is invasive cancer. The presence of cancer may change treatment goals or change the route and timing of delivery. Therefore, colposcopy examination during pregnancy should have as its primary goal the exclusion of invasive cancer. Management of LSIL and HPV-positive ASCUS results during pregnancy should be the same as in the nonpregnant state, although the evaluation of these conditions may be deferred until after delivery.
Management of Cervical Cytology ASCUS and LSIL with HPV-Positive (patients 21 years and older):
Although a cytology result of LSIL is thought to reflect the cytopathic effects of HPV infection rather than a true premalignant lesion, women with LSIL remain at moderate risk for having CIN2+. In ALTS, 27.6% of women with LSIL were found to have CIN2+ either on colposcopically directed biopsies or on close follow-up over the next 2 years (19). This rate is virtually identical to the rate of CIN2+ in women who presented with HPV-positive ASCUS results in the same population (26.7%).
Many studies have shown that prevalence of both HPV DNA positivity and CIN2,3 decreases with age in women with LSIL (19). Well-screened, postmenopausal women with previously negative results are likewise at low risk for invasive cervical cancer. This suggests that postmenopausal women with LSIL may be managed using HPV testing for triage in the same protocol as is used in reproductive-aged women with ASCUS. The presence of ASCUS is less common in postmenopausal women, as is the risk of significant pathologic results. HPV DNA positivity rates also decrease dramatically as women age. This means that HPV testing annually is more efficient in older women because it refers a lower prevalence of CIN2,3 is much higher among women with ASC-H than women with ASCUS, so ASC-H should be considered to represent equivocal HSIL.
Follow-up after colposcopic evaluation of ASCUS, ASC-H, or LSIL with no CIN2,3: In ALTS, the initial colposcopy identified only 58% of the CIN2+ lesions. For the women not found to have CIN2+ at the initial colposcopy, the rate of CIN2+ during follow-up (approximately 10-13%) was unaffected by the findings at colposcopy (negative findings not worthy of biopsy, negative biopsy or CIN1 biopsy). The ALTS evaluated different post-colposcopy follow-up strategies and found that HPV testing performed 12 months after the initial colposcopy and two repeat cytology examinations performed at 6-month intervals are equally effective (19). Because of the additional cost and lack of increased sensitivity, the strategy of combined cytology plus HPV testing is discouraged. In absence of CIN identified histologically, diagnostic excisional or ablative procedures are unacceptable for the initial management of patients with LSIL. Follow-up with either HPV testing at 12 months or cervical cytology at 6 months and 12 months (ASCUS threshold) is acceptable. If the HPV DNA test result is negative or if two consecutive repeat cytology test results are negative, return to routine screening is recommended. If either the HPV DNA test result is positive or if the result of repeat cytology is reported as ASCUS or greater, colposcopy is recommended (24).
Management of Cervical Cytology ASC-H:
The prevalence of CIN2,3 is much higher among women with ASC-H than women with ASCUS, so ASC-H should be considered to represent equivocal HSIL. Women with atypical squamous cell, cannot exclude HSIL (ASC-H) have a 20-50% risk of having a CIN2,3 lesions and should be evaluated with immediate colposcopy. Most women with ASC-H are HPV DNA positive (ranging from 67-84%) (21), so intermediate triage is inappropriate and HPV testing is not recommended. If CIN2,3 is not identified by colposcopy, women aged 21 years and older should be monitored in a manner similar to HPV-positive women with ASCUS.
Management of CIN1 Preceded by HSIL and AGC-not otherwise specific (NOS):
Either a diagnostic excisional procedure or observation with colposcopy and cytology at 6-month intervals for 1 year is acceptable for women 21 years or older with a histology diagnosis of CIN1 preceded by an HSIL or AGC-NOS cytology result, provided in the latter case that the colposcopy examination is satisfactory and endocervical sampling is negative. A diagnostic excisional procedure is recommended for women with CIN1 preceded by an HSIL or AGC-NOS cytology results in whom the colposcopy examination is unsatisfactory, except in pregnancy. The risk of undetected CIN2,3 or an adenocarcinoma in situ (AIS) lesion is expected to be greater in women with CIN1 preceded by an HSIL or AGC cytology result than in women with CIN1 preceded by an ASC or LSIL cytology result. Cervical intraepithelial neoplasia grade 2,3 is identified in 84-97% of women with HSIL cytology results evaluated with a large loop excision of transformation zone (LLETZ) (26). Therefore, separate recommendations are made for CIN preceded by HSIL or AGC cytology.
Integrating HPV Vaccination and Screening:
In a world of limited health resources, HPV vaccination and screening are competing for attention and resources. As proven by large randomized trials, the two licensed HPV vaccines are highly effective at preventing new infections with HPV 16 and 18 (25). There might be some possible cross protection against HPV 31 and HPV45. The minority of cancers are due to other HPV types are not covered. Current vaccines do not treat existing infections or lesions (25). They must be given before exposure; in public health programs this implies that vaccinating young adolescent girls is optimal. It is not supportable to combine HPV vaccination and screening without integration. The proven duration of effectiveness of prophylactic HPV vaccines against HPV 16 and HPV 18 is now approaching a decade and vaccination coverage is increasing. Thus, vaccination of adolescents will largely prevent the first step in cervical carcinogenesis (acute HPV infection in the years after initiation of sexual intercourse); especially as newer vaccines under final evaluation cover more HPV types. The efficacy of all cervical screening tests will decrease due to reduced prevalence of pre-cancers (27). Vaccination especially will reduce efficiency of cytology-based screening because the lesions that remain increasingly will be nuisance look-alikes (particularly ASCUS) not caused by carcinogenic HPV types and not at risk of progression to cancer (28). Thus, various proposals are under consideration, including a delay of initial screening in vaccinated young women and a switch in emphasis from cytology to primary HPV testing.
Summary of Recommendations:
The following recommendations are based on good and consistent scientific evidence and expert opinions for clinical management of patients with abnormal cervical cytology (15)(17):
- In adolescents (before age 21 years) with ASCUS or LSIL cytology results, or CIN1 histology results preceded by ASCUS or LSIL or AGC- not otherwise specific (NOS) cytology results, follow up is recommended at 12-month intervals. At the first follow-up visit (at 12 months), only adolescents with HSIL or greater on the repeat cytology should be referred to colposcopy. At the 24-month follow-up, those with an ASCUS of greater result should be referred to colposcopy. HPV DNA testing is unacceptable for adolescents. If HPV testing is inadvertently performed, a positive result should not influence management.
- Women 21 years and older with either atypical endocervical, endometrial, or glandular cells NOS who do not have CIN or glandular neoplasia identified histologically should receive repeat cytology testing combined with HPV DNA testing at 6 months if they are HPV DNA positive and at 12 months if they are HPV DNA negative. Referral to colposcopy is recommended for women who subsequently test positive for high-risk HPV DNA or who are found to have ASCUS or greater on their repeat cytology tests. If both tests are negative, women can return to routine cytology testing.
- The recommended management of pregnant women with a histology diagnosis of CIN1 is follow-up without treatment. Treatment of pregnant women for CIN1 is unacceptable.
- Women 21 years or older with ASCUS who test negative for HPV, or whose HPV status is unknown and who test negative for abnormalities using colposcopy, should have a repeat cytology test in 1 year. Women with ASCUS who have a repeat cytology test in 1 year. Women with ASCUS who have two negative results on repeat cytology at 6-month intervals can return to routine screening.
- Premenopausal women 21 years and older with ASCUS cytology results may undergo immediate colposcopy or may undergo triage testing to determine which of them should be referred to colposcopy. Triage testing may be performed by a single test by a single test for high-risk (oncogenic) types of HPV or by repeat cytology screening at 6 months and 12 months. When the index cytology test specimen was obtained by liquid-based cytology or when an HPV specimen was co-collected, "reflex" HPV testing is the preferred approach.
- Colposcopy is recommended in premenopausal women 21 years and older with ASCUS who is HPV positive, those with two consecutive ASCUS cytology results or with LSIL, or women of any age with ASC-H.
- In nonpregnant women with ASC and LSIL cytology results who are undergoing colposcopy, endocervical sampling using a brush or curette is preferred for women in whom no lesions are identified and those with an unsatisfactory colposcopy results. Endocervical sampling is acceptable for women with satisfactory colposcopy results and a lesion identified in the transformation zone. Endocervical assessment either with colposcopy or by sampling is recommended for all nonpregnant women with HSIL cytology results. Endocervical curettage is unacceptable in pregnant women.
- In a woman 21 years and older with CIN1 that has persisted for at least 2 years, with continued follow-up or treatment is acceptable. If treatment is selected and the colposcopy result is satisfactory, either excision or ablation is acceptable. If treatment is selected the colposcopy examination is unsatisfactory, the endocervical curettage (ECC) is positive, or the woman has been previously treated, excision is recommended and ablative procedures are unacceptable.
- For premenopausal women 21 years and older with an HPV-positive ASCUS, or ASC-H or LSIL cytology result in whom CIN2,3 is not identified, follow-up without treatment is recommended using either repeat cervical cytology tests at 6 months and 12 months or an HPV test at 12 month-intervals; a repeat colposcopy is indicated for a cytology result of ASCUS or higher-grade abnormality or a positive high-risk HPV test result. After two consecutive negative cytology results or one negative HPV result women can return to routine screening.
- HPV vaccination for females aged 9-26 years. Currently, no changes in screening or management of abnormal cervical cytology are recommended if the adolescent has been vaccinated.
- The 3q26 gain might help identify women with LSIL who do not need colposcopy (30).
Summary
Cervical cancer prevention can now be made even better. Substantial modifications of practice are forthcoming, motivated by improved understanding of HPV natural history and cervical carcinogenesis. Most cervical programs in 2010 still rely on cervical cytology followed by diagnosis of screening-detected abnormalities using colposcopic biopsy. The combined sensitivity of cytology and HPV testing is very similar to HPV testing alone. This indicates that HPV testing might eventually be used as a single primary screening assay followed by cytology or a new biomarker test as a triage to be used only for HPV-positive women. The detection of 3q gain and amplification of TERC in routinely collected Pap smears can assist in identifying low-grade lesions with a high progression risk and in decreasing false-negative cytological screening. The critical challenge is to rethink our current prevention model based on frequent cytology and colposcopic referral. Inevitably, future programs will depend on vaccination, much less frequent screening, and fewer treatments to permit the clearance of resolving HPV infections.
Acknowledgment: Women's Health and Education Center (WHEC) thanks Dr. Robert J. Walat, Clinical Laboratory Director, Ikonisys Inc. New Haven, CT (USA) for very valuable suggestions, expert opinions and assistance with the series on Cervical Cancer Prevention.
Resources
- World Health Organization
Colposcopy and Treatment of Cervical Intraepithelial Neoplasia: A Beginners' Manual - National Cancer Institute
The Pap Test: Questions and Answers
Funding: The series on Cervical Cancer Prevention was funded by WHEC Initiatives for the Global Health. This program is undertaken with the partners of Women's Health and Education Center (WHEC) to eliminate/reduce cervical cancer worldwide. Contact us if you wish to contribute and/or join the efforts.
References:
- Datta SD, Koutsky LA, Ratelle S, et al. Human papillomavirus infection and cervical cytology in women screened in the United States, 200-2005. Ann Intern Med 2008;148:493-500
- American Cancer Society. Cancer prevention and early detection, facts and figures 2006. Atlanta (GA): American Cancer Society; 2006
- ACOG Practice Bulletin. Human papillomavirus, No. 61. Obstet Gynecol 2005;105:905-918
- Wright TC, Massad LS, Dunton CJ, et al. 2006 Consensus Guidelines for the Management of Women with Abnormal Cervical Cancer Screening Tests. 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. Am J Obstet Gynecol 2007;197:346-355
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomized study within organized screening program. BMJ 2010;340:1804-1811
- Arbyn M, Bergeron C, Klinkhamer P, et al. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol 2008;111:167-177
- Saraiya M, Berkowitz Z, Yabroff KR, et al. Cervical cancer screening with both human papillomavirus and Papanicoalaou testing vs. Papanicoalaou testing alone: what screening intervals are physicians recommending? Arch Inter Med 2010;170:977-985
- Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. The Atypical Squamous Cell of Undermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS Group. J Natl Cancer Inst 2000;92:397-402
- Ronco G, Cuzick J, Segnan N, et al. HPV triage of low-grade (LSIL) cytology is appropriate for women over 35 in mass cervical cancer screening using liquid based cytology. Eur J Cancer 2007;43:476-480
- Castle PE, Fetterman B, Poitras N, et al. Relationship of atypical glandular cell cytology, age, and human papillomavirus detection to cervical and endometrial cancer risks. Obstet Gynecol 2010;115:243-248
- Castle PE, Fetterman B, Cox JT, et al. The age-specific relationship of abnormal cytology and human papillomavirus DNA results to the risk of cervical precancer and cancer. Obstet Gynecol 2010;116:76-84
- Rao PH, Arias-Pulido H, Lu XY, et al. Chromosomal amplifications, 3q gain and deletions of 2q33-q37 are the frequent changes in cervical carcinoma. BMC Cancer 2004;4 (www.biomedcentral.com/1471-2407/4/5 )
- Heselmeyer-Haddad K, Janz V, Castle PE, et al. Detection of genomic amplification of the human telomerase gene (TERC) in cytologic specimens as a genetic test for the diagnosis of cervical dysplasia. Am J Pathol 2003;163:1405-1416
- Heselmeyer-Haddad K, Sommerfeld K, White N, et al. Genomic amplification of the human telomerase gene (TERC) in pap smears predicts the development of cervical cancer. Am J Pathol 2005;166:1229-1238
- American Society for Colposcopy and Cervical Pathology (ASCP) Practice Recommendations. Available from http://www.asccp.org/edu/practice/cervix/premalignant/epidemiology.shtml Accessed 10 August 2010
- Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet 2007;370:890-907
- American Society for Colposcopy and Cervical Pathology. Management of women with atypical squamous cells of undetermined significance (ASCUS). Hagerstown (MD): ASCCP; 2007. Available at: http://www.asccp.org/pdfs/consensus/algorithms_cyto_07.pdf Accessed 2 August 2010
- Moscicki AB, Shiboski S, Hills NK, et al. Regression of low-grade squamous intraepithelial lesions in young women. Lancet 2004;364:1678-1683
- Guido R, Schiffman M, Solomon D, et al. Post-colposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undermined significance: a two-year prospective study. ASCUS LSIL Triage Study (ALTS) Group. Am J Obstet Gynecol 2003;188:1401-1405
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 Consensus Guidelines for the Management of Women with Abnormal Cervical Screening Tests. 2006 ASCCP-Sponsored Consensus Conference. J Low Genit Tract Dis 2007;11:201-222
- Sherman ME, Castle PE, Solomon D. Cervical cytology of atypical squamous cells-cannot exclude high-grade squamous intraepithelial lesion (ASC-H): characteristics and histologic outcomes. Cancer 2006;108:298-305. (Level I)
- Wright TC Jr, Schiffman M, Solomon D, et al. Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol 2004;103:304-309. (Level III)
- Kjaer S, Hogdall E, Frederiksen K, et al. The absolute risk of cervical abnormalities in high-risk human papillomavirus-positive, cytologically normal women over a 10-year period. Cancer Res 2006;66:10630-10636. (Level II-2)
- ACOG Practice Bulletin. Management of abnormal cervical cytology and histology. Number 99; December 2008
- Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16-18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomized study in young women. Lancet 2009;374:301-314
- Alvarez RD, Wright TC. Effective cervical neoplasia detection with a novel optical detection system: a randomized trial. Optical Detection Group. Gynecol Oncol 2007;104:281-289. (Level I)
- Fanco EL. Cuzick J, Hildesheim A, et al. Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine 2006;24 Suppl 3:S171-177
- Shiffman M, Wentzensen N. From human papillomavirus to cervical cancer. Obstet Gynecol 2010;116:177-185
- Seppo A, Jalali GR, Kilpatrick MW, et al. Gain of 3q26: A genetic marker in low-grade squamous intraepithelial lesions (LSIL) of the uterine cervix. Gyncol Oncol 2009;114:80-83
- Jalali GR, Herzog TJ, Dziura B, Walat RJ, et al. Amplification of the chromosome 3q26 region shows high negative predictive value for nonmalignant transformation of LSIL cytologic finding. Am J Obstet Gynecol 2010;202:1.e1-e5
Published: 5 November 2010
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com