Cervical Cancer Prevention: Managing High-Grade Cervical Neoplasia
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Cervical cytology screening programs are associated with a reduction in the incidence of and mortality from invasive squamous and non-squamous cancer. Given that infections with the human papillomavirus (HPV) can lead to cervical cancer, screening and diagnostic programs involving Papanicolaou smears (Pap test) and colposcopy are the standard of care in North America. As more than 80% of cervical cancers are preventable by routine screening, the United States has clearly been successful in reducing HPV-related cancers with the implementation of the cervical cancer screening programs. In developing countries, where the implementation of cervical cancer screening programs can be challenging, the rates of cervical cancer are noteworthy. For example, there were 87,466 cases of cervical cancer in the developed world in 2007 vs. 473,430 cases in the developing world (1). However, despite the fact that careful screening can help to reduce the rate of cervical cancer, improvements in the triage of abnormal Papanicolaou smears are still needed worldwide. The system of triaging cytological and histological cervical abnormalities is frequently being revised; the actual colposcopic examination is less frequently challenged. Because only a small percentage of cervical dysplasia progresses to cancer, research is headed towards the development of alternative methods for identifying patients at highest risk for disease progression. Improvements should be focused on recognizing high-risk features during colposcopy, and adequately identifying those patients at risk for progression of disease during the follow-up period.
The purpose of this review is to discuss the current management and summarize recommendations for managing high-grade cervical neoplasia (CIN2, 3+). Areas in which improvement can be made in the recognition of high-risk features during colposcopy are also discussed. Once colposcopic technique is improved, accuracy for detection of high-risk premalignant disease increases. Carcinogenic or "high-risk" human papillomavirus (high-risk HPV) testing has become the standard triage worldwide for women with atypical squamous cells of undermined significance (ASC-US) cytology and is designated as a stand-alone follow-up option in a number of post-colposcopy and post-treatment clinical management scenarios.
Abbreviations Used
Adenocarcinoma in situ -- AIS
American Cancer Society -- ACS
American College of Obstetricians and Gynecologists -- ACOG
American Society for Colposcopy and Cervical Pathology - ASCCP
Atypical glandular cells -- AGC
Atypical squamous cells -- ASC
Atypical squamous cells of undermined significance -- ASC-US
Atypical squamous cells, cannot exclude HSIL -- ASC-H
ASC-US LSIL Triage Study -- ALTS
Cervical intraepithelial neoplasia (mild or grade 1) -- CIN1
Cervical intraepithelial neoplasia, (moderate or grade 2) -- CIN2
Cervical intraepithelial neoplasia, (severe or grade 3) -- CIN3
Large Loop Excision of Transformation Zone -- LLETZ
Low-grade squamous intraepithelial lesion -- LSIL
High-grade squamous intraepithelial lesion -- HSIL
Human papillomavirus -- HPV
Background
The mean reporting rate of high-grade intraepithelial lesion (HSIL) in U.S. laboratories is 0.7% (2). The rate of HSIL varies with age. The cytology result of HSIL carries a high risk of significant cervical disease. The prevalence of CIN3 peaks between ages 25 years and 30 years, and progression to cancer usually takes at least a decade longer (3). Severe cervical intraepithelial neoplasia 3 (CIN3) is almost invariably caused by high-risk HPV, and women are generally high-risk HPV positive for several years before a CIN3 diagnosis. However, no test has 100% accuracy, and HPV testing is no exception. The key to developing effective guidelines for the management of cervical abnormalities is to distinguish true cervical cancer precursors from benign cervical abnormalities with premalignant potential in a cost effective manner. As many as 28% of women with cytologic low-grade intraepithelial lesion (LSIL) harbor CIN2 or CIN3, approximately two thirds of which is identified by colposcopy (2). High risk HPV types 16 and 18 are responsible for 70% of squamous cell carcinoma cases and 86% of adenocarcinoma (2). This is disproportionately different for CIN2 and CIN3 with only about 50% of lesions being associated with HPV types 16 and 18. Persistent HPV infection and cervical intraepithelial neoplasia 3 (CIN3) allow random mutations to accumulate that can eventually lead to cancer. When mutations occur in normal cells, the cell upregulates the expression of proteins that repair the mutation or precipitate apoptosis. High-risk types of the HPV virus produce proteins that inhibit this process, allowing cells to live despite mutations. In the worst case, these cells continue to grow and mutate, forming a malignancy over many years that invades the basement membrane. Prophylactic administration of HPV vaccines effectively prevents the development of CIN1, 2, 3, and adenocarcinoma in situ (AIS). In patients who are tested negative for the vaccine HPV types during the administration of the 3-dose vaccine, protection against high-grade cervical lesions (CIN2 and 3) and AIS was between 97% and 100% at 3 years (3). Vaccination is also effective in eliminating the development of genital warts and CIN1, which are caused by low-risk HPV types 6 and 11 (3). Additionally, vaccination appears to offer cross-protection, i.e., partial protection against infection with HPV types related to 16 or 18, such as high-risk types 45, 31, 33, and 52 (4). Two effective HPV vaccines are commercially available. Direct comparisons concerning looking at clinical outcomes are not available but important differences in immunogenicity and cross-protection between products are likely.
Natural History of Human Papillomavirus (HPV) Infection
HPV is the most common sexually acquired infection in the world. Numerous natural studies (5) have demonstrated that as many as 50% of sexually active young women in the United States will have positive test results for HPV within 36 months of their sexual debut. Recurrent infections also are common. Consequently, prevalence data indicate that up to 57% of sexually active female adolescents in the United States at any one point in time are infected with HPV (3). The cytopathic effects of HPV can be detected by the Pap test. Natural history studies of adolescents with newly acquired HPV infection show that HPV usually becomes undetectable after an average of 8 months. In most adolescent patients with an intact immune system, most HPV infections will resolve within 24 months (5). Spontaneous regression of CIN2 and CIN3 is also associated with viral clearance, but it rarely occurs. The duration of HPV positivity is shorter and the likelihood of clearance is higher among younger women (5).
The presence of high-risk HPV is a marker for the risk of diagnosis of CIN2,3+; only 1 in 10 to 1 in 30 HPV infections are associated with abnormal cervical cytology results, with an even smaller proportion associated with CIN2,3+ (6). Among women with negative cytology test results and a positive HPV test result, only 15% will have abnormal cytology results within 5 years (6). However, high-risk HPV is necessary for the development and maintenance of CIN3. Persistent high-risk HPV is a necessary but not sufficient condition for the development of almost all types of invasive cervical cancer. Conversely, the risk of cervical cancer in women who do not harbor oncogenic HPV is extremely low (<1%). The longer high-risk HPV is present and the older the patient, the greater risk of CIN. When HPV is present, smoking doubles the risk of progression to CIN3 (7). Although expression of the presence of HPV as CIN can occur within months of viral acquisition, the time course from CIN3 to invasive cancer averages between 8.1 years and 12.6 years (7)(8). The slow pace of these changes in immunocompetent women means that accurate estimates of progression risk require long follow-up periods. Perhaps more relevant for clinical practice are estimates of regression to normal status. A review of literature from 1950 to 1992 noted the likelihood of regression to be 60% for CIN1 and 40% for CIN2 (7).
n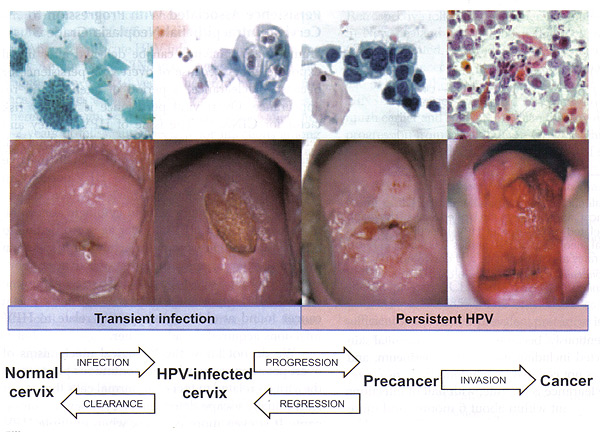
Figure 1. Cervical cancer progression model.
The stages in cervical carcinogenesis: With the exception of very rare HPV-negative cases, cervical cancer arises via clear necessary steps -- acute infection with carcinogenic HPV type(s), viral persistence (rather than clearance) linked to development of cervical precancer, and invasion. There are three important peaks in the natural history of HPV and cervical cancer (9) -- the ages at which the peaks occur vary by region, and can be timed for the average age at first sexual intercourse for that region, because HPV infection "starts the clock" for the events that follow. A secondary peak of precancer (here including CIN2 and CIN3) 5-15 years later (the timing is dependent on the intensity of screening and precancer diagnosis); and then a long drawn-out peak or plateau in invasive cancers decades later. The average time between HPV incidence and CIN3 development is much shorter than the time between CIN3 and progression to invasion (10). Notably, new infections at older ages are just as likely to clear as in younger women. Because of the overall time required to move from HPV to invasive cervical cancer, new infections acquired at older ages contribute little to cervical cancer in populations. Overt viral persistence is the key risk factor for CIN3.
Potential factors affecting the progression of oncogenic HPV (28):
Infection Normal → Cervix ← Clearance |
ASC-US | CIN1 | CIN2 | CIN3 | Invasive Cervical Cancer |
| Oncogenic HPV-infected cervix -age -mature cervix -microabrasion of the cervix -suppressed immune system -co-infection with other microorganism -smoking -number of sexual partners |
Persistence → ← Regression -HPV 16-18 -Response of immune system -co-infection with other microorganism -use of oral contraceptives? |
Precancer -HPV 16/18 -response of immune system |
Invasion → -HPV 16/18 -increasing age -suppressed immune system -co-infection with other microorganisms |
Colposcopy to Evaluate Abnormal Cervical Cytology
CIN3 lesions are usually within the transformation zone; the most severe disease is found at the most proximal (cephalad) extent of lesion. CIN3 may extend into the endocervical canal; usually a single lesions; may be located alongside or within low-grade lesions; and varies in size. Larger lesions tend to correlate with the severity of disease and the risk of occult invasion as well as the risk of treatment failure. The following descriptions refer to the colposcopic appearance of CIN3 lesions after the application of acetic acid.
Margins: Sharply demarcated lesion edges, often with very straight contours; lack the geographic, feathered, or indistinct margins. CIN3 often coincides with and may be difficult to distinguish from a larger CIN1 or CIN2 lesion. Internal margins (borders) describe abrupt change in the nature of a lesion(s) as the examining eye moves radially from outer to inner (proximal) within the transformation zone (TZ). A so-called "lesion within a lesion" or "border within a border" is a feature of high-grade dysplasia, with the inner, more proximal lesion being more severe. Severe lesions have raised, rolled, or peeling margins.
Color: Distinct, denser aceto-whitening are associated with high-grade lesions. Low-grade lesions contain a dull surface due to increased nuclear density and less reflection of the incident light. Finally, a dull or gray-white to oyster gray color and a more prompt and persistent acetic-white change is a clinical feature of CIN3.
Vascular Pattern: Vascularity is the best predictor of lesion severity including the presence of invasion. For example, CIN3 lacks the fine vascular patterns typical of low grade lesion. "Coarse" vascular patterns (punctation, mosaicism, or both) characterized by larger and varied caliber of vessels; larger and variable intercapillary distances; "umbilicated" mosaic patterns, with punctation in the middle of the "tiles" suggests CIN 3. Vascular patterns can be striking and visible even at lower magnification; vascular patterns change as acetic acid effects develop, and then fade. Prominent and dilated vessels may have blunt acetowhite changes. It is common to miss the CIN3 or invasive cancer because the examining eye is drawn to acetowhite changes and away from the less-white high-grade abnormalities.
n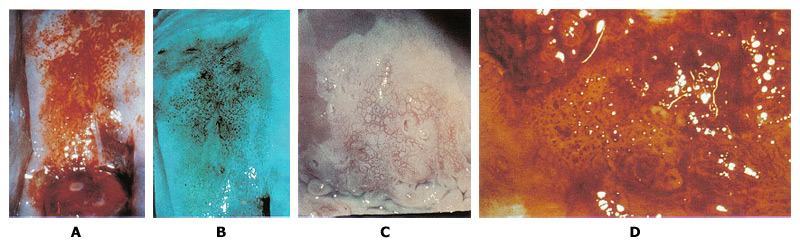
Figure 2. A. Punctation on the anterior lip of the cervix is mixed with some white epithelium. Biopsy specimen for the area showed CIN3. B. Use of green filter on the colposcope enhances the appearance of punctation. C. Mosaic pattern is seen within the white epithelium. Biopsy from the area showed carcinoma-in-situ. D. Atypical vessels. The terminal vessels are irregular in size, shape and arrangement. Biopsy specimens revealed invasive carcinoma.
Histology of CIN3: Cellular maturational abnormality extends into the upper third of the epithelial thickness; basement membrane intact. Cellular and especially nuclear hyperchromasia and pleomorphism; some mitoses may be seen.
n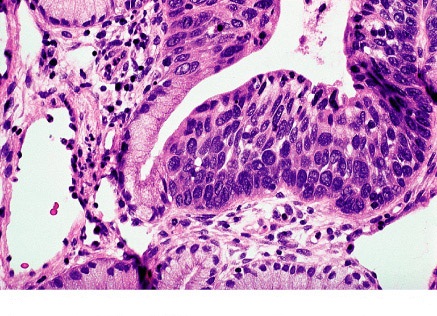
Figure 3. HSIL histology: extensive involvement by CIN3 of surface epithelium and glands of cervix.
The Reid Colposcopic Index (RCI): Despite its questionable accuracy RCI has been helpful in developing a colposcopic impression, and determining where to biopsy. While colposcopic impression is based on a subjective reading of the gross cervical appearance, the impression is traditionally assessed and reported using a scoring system called the RCI. The RCI considers 4 colposcopic signs: margin, color, vessels, and iodine staining. Each sign is scored between 0 and 2, with 2 indicating advanced dysplasia or CIN3. The scores are summed, with higher numbers being more suggestive of dysplasia. An abbreviated RCI scoring was recently described and involves 3 of the 4 signs, eliminating Lugol's iodine staining, which has largely been excluded by colposcopists (11). This scoring system attempts to standardize the subjective nature of impressions. Of interest, digital images are less accurate than the live colposcopic examinations. Unfortunately, the RCI impression is often in contrast to the biopsy results and can thus present a challenge for treatment and follow-up. The challenge relates to the low sensitivity of colposcopic impression: 74% and 90.7% respectively (12). Clearly, the number of biopsies, regardless of the RCI, increases the sensitivity of colposcopy in identifying CIN3. In addition, even when high-grade dysplasia is absent after a colposcopic evaluation of an HSIL Pap smear, loop excision still identifies CIN3 in a significant number of cases potentially obviating colposcopy all together when a Pap test shows HSIL.
| The Reid Colposcopic Index (RCI) | ||||
| Modified RCI | Margin | 0 point | 1 point | 2 points |
| -Condylomatous -Feathered margins -Angular, jagged -Satellite lesions -Extend beyond TZ zone |
-Regular, smooth, straight |
-Rolled, peeling -Internal demarcations |
||
| Color | -Shiny, snow- white indistinct |
-Shiny gray | -Dull, oyster-white | |
| Vessels | -Fine-caliber -Poorly formed patterns |
-Absent vessels | -Punctation or mosaic | |
| Iodine | -Positive staining -Minor iodine negativity |
-Partial uptake | -Negative staining of significant lesion | |
| Score | = 0-2 CIN1 | = 3-4 CIN1 or 2 | = 5-8 CIN 2-3 | |
New techniques: Only a small percentage of cervical dysplasia progress to cancer, research is headed toward the development of alternative methods for identifying patients at highest risk for disease progression. The high false-negative and false-positive rate of Papanicolaou (Pap) test alone suggests the need for an adjunct to the Pap test (perhaps in addition to HPV testing) or to the pathological evaluation of the colposcopy-guided biopsy to improve specificity. This would consequently help reduce referrals for follow-up colposcopy, because thus far, colposcopy is an imperfect means of predicting risk. In one study (13) it was reported that 60% of CIN1 lesions regressed, 10-15% progressed to CIN2-3, and only 0.3% progressed to invasive cancer. Others have reported that only 1.9% of women with CIN1 will develop cancer in 1-2 years. Clearly some patients with CIN1 are at risk of progression and methods of identify them are needed (14). Several methods to predict risk are currently under study, and 3 such tests are discussed here: the use of p16, deoxyribonucleic acid (DNA) methylation, and optical spectroscopy. A tumor suppressor gene, p16, is a cell cycle regulator. There have been multiple reports of overexpression of p16 in dysplasia and cancer of cervix; and p16 can be used to improve the histological diagnostic accuracy of CIN3 (15). The staining is typically scored by the pathologist as 0 (none), 1 or greater, and 2 or greater, depending on the degree of intensity. The sensitivity and specificity of p16 for diagnosis of CIN3 in the combined group are 96% and 83% respectively (15). In terms of agreement among pathologists, the addition of p16 testing improved the interobserver agreement in final histologic diagnosis. Although, reported to be a cost-effective marker, a recent review of the literature documented the pitfalls in immunohistochemistry and the interpretation of p16 staining in this setting (16).
DNA methylation serves to silence the transcription of certain genes. In some recent studies, investigators hypothesized that methylation of certain genes in cervical dysplasia may indicate a higher risk for progression and the development of cancer. For example, the silencing of a tumor suppressor gene, cell adhesion molecule 1, through methylation of its promoter, may be more frequently observed in high-grade cervical dysplasia and cancer (17). In an observational study of 171 patients, methylation increased from 5% to 30% in CIN3 lesions and 83% in squamous cell carcinomas (17).
Finally, techniques in optical spectroscopy have been developed and tested to aid in improving the diagnostic accuracy of colposcopy. Optical spectroscopy is a real-time diagnostic method that can be used along with colposcopy. Although spectroscopy appears add little to colposcopy, one study (18) suggests that spectroscopy might serve as an adjunct to colposcopy. This technique has been reported to be well accepted by the patients and providers, as well as cost effective, although the appropriate use of this technology has yet to be documented and implemented clinically (18).
Endocervical Sampling
Endocervical sampling may be conducted either with vigorous endocervical brushing or by traditional endocervical curettage (ECC) with a sharp curette. It is not indicated in the pregnant patients. Sampling should be performed if colposcopy results are unsatisfactory or if ablative treatment, such as cryotherapy or laser ablation, is contemplated. In efforts to improve sensitivity, many colposcopists add endocervical sampling to biopsy. In the analysis of the ASC-US LSIL Triage Study (ALTS) trial, only 3.7% of 1119 colposcopic examinations yielded abnormal ECCs (>CIN2) vs. 21.7% with CIN2 or greater on colposcopy-directed biopsy (19). The sensitivity of biopsy is thought to improve with the addition of an ECC in women older than 40 years of age. For example, in ALTS, the risk of not detecting CIN2 or greater by not performing ECC was only 7 of 653 women (1.1%) in all ages. However, in women 40 years old and older, the addition of ECC improved the sensitivity of detecting CIN2 or greater. In women younger than 40 years, 7 of 312 women (2%) had CIN2 or greater on ECC with less than CIN2 diagnosed on biopsy. However, in women 40 years old or older, 3 of 23 women (13%) had CIN2 or greater on ECC with less than CIN2 diagnosed on biopsy (19). In other words, of those with CIN2 or greater at initial colposcopy, only 10 of 253 were diagnosed by ECC alone: 7 in women younger than 40 years old and 3 in women 40 years old or older. Although there may be a slight improvement in diagnosis of CIN2 or greater with the addition of ECC, it may be difficult to justify the cost effectiveness of this procedure. In women with ASC-H, HSIL, AGC or AIS cytology results, ECC should be considered as part of initial colposcopy examination.
Large Loop Excision of the Transformation Zone (LLETZ) and Cone Biopsy
LLETZ became the instrument of choice in the 1990s and has gained a tremendous experience in USA. It appears to be the current treatment of choice for high-grade dysplasia even with very limited follow-up. Many physicians are reluctant to use LLETZ in the young, nulliparous patients because the cervix is small and a considerable amount of the cervix can be removed with this procedure. Thermal artifact, although reported in many early series, is now of minimal concern, (20). This is probably related to equipment power setting and technical problems such as "stalling". Side effects are mainly secondary to hemorrhage (initially reported at 10% but with experience found to be in the 1-2% range). Long-term side effects such as those on pregnancy rates and preterm births are similar to those following laser vaporization or electrocoagulation (22).
Conization of cervix: After the extent of involvement of epithelium on the cervix has been clearly demarcated by colposcopy, the limits of the base of the cone biopsy on the cervix can be determined. An incision (cold-knife or LLETZ) that is certain to include all the abnormal areas is made into the ectocervix. This incision does not need to be circular but should accommodate excision of all atypical epithelium. The depth of the incision as it tapers toward the endocervical canal should be determined by the length the cervical canal and the suspected depth of involvement. Often the entire limits of the lesion have been visualized, and very shallow conization is sufficient. Cervical conization does not need to be a fixed technical procedure for all patients, but it should always be individualized and consist of adequate excision of all involved areas. Bleeding from the cone bed can usually be controlled by electrocauterization and by placing Monsel's paste on the base. The use of Sturmdorf sutures is probably unnecessary in most cases. Significant cervical stenosis, cervical incompetence, or infertility with cervical factors is rare complication.
Management of High-Grade Intraepithelial Lesions (HSIL)
High-Grade Cervical Intraepithelial Neoplasia2, 3 in Adolescents (before age 21 years)
CIN2 is a significant abnormality that has classically required therapy. A variety of studies, including the ALTS trial, have demonstrated that this lesion may have a significant rate of resolution (up to 40% in adults) (8). This rate of resolution is suspected to be higher in adolescents. In the 2006 consensus guidelines the management of CIN2, 3 in adolescents (20 years and younger) and young women in observation with colposcopy and cytology at 6-month intervals for up to 24 months or treatment with either ablation or with excision of the transformation zone (21). When CIN2 is specified, observation is preferred for the adherent patient. When CIN3 is specified or if colposcopy confirmation is unsatisfactory, treatment is recommended. If the colposcopic appearance of the lesions worsens, or if the high-grade cytology or colposcopy persists for 1 year, a repeat biopsy is warranted. Treatment is recommended for the patient with persistent CIN2, 3 by histology for a 24-month period. If CIN1 is found, continued observation is an option. CIN3 is a significant cervical abnormality. Despite the fact that cervical cancer is very rare in the adolescent population, the natural history of CIN3 in this population has not been examined. Therapy is recommended for all women with CIN3 (22). Some authors have recommended that excision be used for the management of biopsy-confirmed CIN3, especially for large lesions that are at increased risk of having microinvasive or occult invasive carcinoma. The type of intervention should be based on the geometry of the cervical lesion as well as the clinical recommendations of the health care providers.
HSIL Cervical Cytology in Adults (after age 21 years)
A single colposcopy examination identifies CIN2+ in 53-66% of women with HSIL, and CIN2+ is diagnosed in 84-97% of women evaluated with LLETZ (19)(23). Traditionally, the management of HSIL cytology results has relied on the colposcopy identification of high-grade CIN, followed by treatment when lesions are found. This strategy has proved to be highly successful in reducing cervical cancer rates in developed countries. Because colposcopy can miss a significant number of CIN2, 3 lesions and most women with HSIL will eventually undergo a diagnostic excisional procedure, a single-visit strategy (see and treat) is attractive in women in whom future fertility is not an issue. This strategy has been shown to be feasible and cost effective (7). A diagnostic procedure is also recommended for women with HSIL in whom the colposcopy examination is unsatisfactory, except in pregnant women. Because of the limited accuracy of colposcopy generally and of colposcopy grading particularly, colposcopy assessment is no longer required before immediate LLETZ. Nevertheless, prudence would suggest that colposcopy is helpful to tailor the excision to the size of the lesion and the limits of the transformation zone.
An important consideration before treatment should be whether the high-grade cytology result is due to a vaginal lesion. Careful examination of the vagina using 3-5% acetic acid may reveal a high-grade vaginal lesion. In such case, although the cervix has no lesion, the cytology result is correctly positive, and the patient's disease can be cleared with appropriate therapy. Application of Lugol's solution to the cervix is controversial and frequently omitted as it adds little to the examination. The predictive value of an HSIL cytology result is limited, and some women with HSIL have CIN1, subclinical HPV infections without colposcopically visible lesions, or even no disease. Cytology interpretation is subjective, and women with HSIL diagnoses may not have HSIL. In a study of the reproducibility of cervical cytology, 27% of women with HSIL were found to have LSIL on review of their slides, whereas 23% had ASCUS, and 3% had negative results (24). Therefore, both the possibility of missed disease and the potential for overtreatment must be considered, and the management must be individualized based on the patient's needs. When CIN2,3 is not identified histologically, either a diagnostic excisional procedure or observation with colposcopy and cytology at 6 months and 12 months is acceptable, provided in the latter case that the colposcopy examination is satisfactory and ECC is negative. If observation with cytology and colposcopy is elected, a diagnostic excisional procedure is recommended for women with the results of HSIL on repeat cytology at either the 6-month or 12-month visit. After 1 year of observation, women with two consecutive negative cytology results can return to routine screening. Ablation is unacceptable when CIN2,3 is not identified histologically or ECC identified CIN of any grade (22).
Management of CIN2 and CIN3
CIN3 generally is considered to be a cancer precursor, although not all CIN3 lesions will progress to cancer. The risk of progression of CIN3 is unclear because most experts consider risk too high to justify observation. A biopsy diagnosis of CIN3 may miss occult invasive cancer and apparent progression after a colposcopy biopsy diagnosis may reflect missed prevalent cancer. CIN2,3 lesions associated with HPV 16 genotype are less likely to regress, as are those in women with the HLA 201 phenotype (25). The significance of CIN2 is unclear. The risk of progression to CIN3 and cancer appears greater for women with CIN2 than for women with CIN1. However, many women with CIN2 will have regression of their lesions without therapy. No accepted tests are available to distinguish CIN2 that reflects and exuberant HPV infection from that with true malignant potential. The cutoff between CIN1 and CIN2 and between CIN2 and CIN3 is arbitrary. Because of the moderate cancer risk associated with CIN2, the decision among leaders in colposcopy and cervical cancer prevention in the United States has been to consider CIN2 the threshold for treatment for most U.S. women.
All pregnant women with HSIL should undergo colposcopy. The goal of cytology and colposcopy during pregnancy is to identify invasive cancer that requires treatment before or at the time of delivery. However, unless cancer is identified or suspected, treatment of CIN is contraindicated during pregnancy. CIN has no effect on the woman or fetus, whereas cervical treatments designed to eradicate CIN can result in fetal loss, preterm delivery, and maternal hemorrhage. ECC is contraindicated during pregnancy. Colposcopy during pregnancy can be challenging because of cervical hyperthermia, the development of prominent normal epithelial changes that mimic preinvasive disease colposcopically, obscuring mucus, contact bleeding, prolapsing vaginal walls, and bleeding after biopsy. Biopsy is important if the colposcopy impression is high grade, especially in older pregnant women at higher risk of invasive cancer. Once cancer has been excluded, cervical therapy can be deferred until postpartum. CIN2,3 rarely progresses to invasive cancer during the few months of pregnancy. For these reasons, observation of pregnant women appears a safe and reasonable approach, provided cancer has been ruled out.
HIV-Positive women with CIN2 and CIN3: Standard ablative or excisional treatment is recommended for women who are HIV positive with documented CIN2 or CIN3, regardless of HIV viral load. Effective treatment of CIN requires immunologic clearance of suppression of HPV to avoid recurrence (7). Women who are HIV-positive have difficulty clearing HPV, and therefore are at increased risk of recurrent disease in direct relation to their level of immunosuppression. Treatment of CIN should be pursued despite high recurrence rates (greater than 50% recurrence rate after standard treatment) because it can effectively interpret progression to invasive cancer (26). Women who are HIV-positive also appear more likely to have positive surgical margins, which may contribute to increased recurrence rates (26). Because recent studies reported a lower prevalence of high-grade disease and HPV DNA positivity among immunosuppressed women, the 2006 consensus guidelines recommend that the management of these conditions be similar to that in the general population. The role of highly active antiretroviral therapy in the management of precancerous cervical lesions remains unclear. Therefore, CIN2 and CIN3 should be treated similarly in women who are HIV-positive regardless of their use of antiretroviral therapy. In this analysis (29) of covariance models, higher baseline score, younger age, higher education level, higher income, and former- as opposed to never-drug users, but not HIV status, were associated with improved knowledge. High-risk women's understanding of cervical cancer and HPV has improved, but gap remains (29). Improvement has been weakest for less educated and lower-income women.
Role of Hysterectomy in Management of CIN2,3+
Hysterectomy in the absence of other indications, such as abnormal bleeding or uterine leiomyomas, usually is not required. However, one indication is in a patient with recurrent disease when the residual cervix is too small to allow safe repeat conization without risk of bladder and vaginal injury. A repeat diagnostic excision or hysterectomy is acceptable for women with a histology diagnosis of recurrent or persistent CIN2,3. If excision is indicated, it should be performed (where possible) before hysterectomy to rule out invasive cancer. If hysterectomy is performed, the choice of either vaginal or abdominal approach should be dictated by other indications, such as surgeon's experience and patient characteristics and preferences.
Summary of Recommendations
The following recommendations are based on good and consistent scientific evidence and expert opinions for clinical management of patients with abnormal cervical cytology (27):
- In adolescents (before age 21 years) with HSIL cytology results, a satisfactory colposcopy result, negative ECC and no CIN2,3 identified on colposcopy biopsy, follow-up is recommended at 6-month intervals with Pap testing and colposcopy for up to 24 months. If during follow-up a high grade colposcopy lesion is identified or HSIL cytology results persist for 1 year, biopsy is recommended. If HSIL persists for 24 months without identification of CIN2,3, or if the colposcopy result is unsatisfactory, a diagnostic excisional procedure is recommended. After two consecutive negative results, women can return to routine cytology testing.
- For adolescents and young women with a histology diagnosis of CIN 2,3 not otherwise specified (NOS) and a satisfactory colposcopy result either treatment or observation for up to 24 months using both colposcopy and cytology at 6-month intervals is acceptable. When a histology diagnosis of CIN2 is specified, observation is preferred. When histology of CIN3 is specified or when the colposcopy results are unsatisfactory, treatment is recommended. If the colposcopy appearance of the lesion worsens or if an HSIL cytology result or a high-grade colposcopy lesion persists for 1 year, repeat biopsy is recommended. After two consecutive negative cytology results, women with normal colposcopy results can return to routine cytology screening. Treatment is recommended if CIN3 is subsequently identified or if CIN2,3 persists for 24 months.
- In women 21 years and older with HSIL cytology results, immediate loop electrosurgical excision of transformation zone or colposcopy with ECC are both acceptable management options. In adolescents and pregnant women with HSIL cytology results, colposcopy is recommended. Immediate excision is not acceptable in adolescents and pregnant women. A diagnostic excisional procedure is recommended for all pregnant women with HSIL when colposcopy is unsatisfactory or when CIN of any grade is identified on ECC.
- Post-treatment management options for women 21 years and older who have CIN2,3 include a single HPV DNA test at 6-12 months, cytology alone at 6-month intervals or a combination of cytology and colposcopy at 6-month intervals. For adolescents who have undergone treatment, cytology follow-up is preferred. Colposcopy with ECC is recommended for women who are HPV DNA positive or have a result of ASC-US or greater on repeat cytology. If the HPV DNA test is negative or if two consecutive repeat cytology test results are negative, routine screening commencing at 12 months is recommended for at least 20 years.
- For women 21 years and older, the preferred management of CIN2,3 identified at the margins of a diagnostic excisional procedure or in an ECC obtained at the end of the procedure is reassessment using cytology with ECC at 4-6 months following treatment. Performing a repeat diagnostic excisional procedure is acceptable, as is a hysterectomy if a repeat diagnostic procedure is not feasible for women with histology diagnosis of recurrent or persistent CIN2,3.
- In nonpregnant women 21 years and older, both excision and ablation are acceptable treatment modalities in the presence of histology diagnoses of CIN2,3 and satisfactory colposcopy result. Ablation is unacceptable when colposcopy has not been performed, the ECC is positive for any grade of CIN, the colposcopy result is unsatisfactory, or a woman has recurrent CIN2,3.
- In nonpregnant women 21 years and older with HSIL in whom CIN2,3 has not been identified; three management options are acceptable: diagnostic excisional procedure; review of cytology, histology and colposcopy findings and management of the patient according to the revised interpretation; or if the colposcopy findings and management of the patient according to the revised interpretation; or if colposcopy is satisfactory and ECC is negative, observation with colposcopy and cytology at 6 months-intervals for 1 year. A diagnostic excisional procedure is recommended for women with repeat HSIL cytology results at either the 6-month or 12-month visit. Women with two consecutive cytology results can return to routine screening.
Summary
The improved sensitivity of cytological screening, along with the addition of HPV testing, could potentially lead to large numbers of referral to colposcopy. However, the actual technique of colposcopy has yet to be improved. Although the protocol of combined Pap smear and colposcopy will rarely miss a squamous cell carcinoma, there are recent concerns that a large number of unnecessary colposcopy and follow-up procedures are being performed for low-risk disease. Improvements should be focused on recognizing high-risk features prior to colposcopy, properly identifying high-risk features during colposcopy, and adequately identifying those patients at risk for progression of disease during the follow up period. Areas in which we might improve the recognition of high-risk features during colposcopy are identified in this review. Currently, the best means to identify lesions worthy of biopsy include adequate visualization of the cervix by using methods to reduce anxiety, pain, and bleeding, as well as updating the evidence regarding the accuracy of acetic acid and/or Lugol's solution in the colposcopic setting. The sensitivity of colposcopy for the identification of prevalent CIN3 has been estimated between 60% and 80%. It is demonstrated that a better performance in detecting prevalent precancer is related to the number of biopsies taken rather than the experience of colposcopist. The diagnosis of CIN3 carries a risk of cervical cancer developing over the next 9-12 years in about 12-36%. Unlike CIN1 and CIN2, the chance of spontaneous regression, even in an immunocompetent woman, is small. For this reason, CIN3 should be treated with either an ablative procedure or an excisional conization of cervix. Cervical cancer prevention efforts must be adapted to the resources available in each region, and the local tolerance for residual risk, knowing that no program will prevent every case. In rich countries striving for the very lowest rates of cervical cancer, such as in the United States, the challenge is to use new technology but to avoid excessive interventions and overtreatment. At present, HPV is approved as an adjunct to cytology, performed in women 30 years and above. The great burden of cervical cancer is in low-resource regions. The advent of very low cost, accurate HPV tests has finally produced a practical possibility of extending sustainable screening once or twice per lifetime to poor countries.
Acknowledgement: Gratitude is expressed to Bradley J. Monk, MD FACS FACOG, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Comprehensive Cancer Center, Creighton University School of Medicine at St. Joseph's Hospital and Medical Center, a member of Catholic Healthcare West, Phoenix, AZ (USA) for the expert opinions and preparation of the manuscript.
Funding: The series on Cervical Cancer Prevention was funded by WHEC Initiatives for the Global Health. This program is undertaken with the partners of Women's Health and Education Center (WHEC) to eliminate/reduce cervical cancer worldwide. Contact us if you wish to contribute and/or join the efforts.
References
- Garcia M, Jamal A, Ward EM, et al. Global cancer facts and figures 2007. Atlanta, GA: American Cancer Society; 2007
- Castellsague X, Diaz M, de Sanjose S, et al. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst 2006;98:303-315
- Garland SM, Hernandez-Avila M, Wheeler CM, et al. Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007;356:1928-1943
- Paavonen J, Jenkins D, Bosch FX, et al. HPV PATRICIA study group. Efficacy of a prophylactic adjuvant bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomized controlled trial. Lancet 2007;369:2161-2170
- Winer RL, Lee SK, Hughes JP, et al. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemol 2003;157:218-226
- Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. N Engl J Med 2003;348:518-527. (Level II-2)
- ACOG Practice Bulletin. Management of abnormal cervical cytology and histology. Obstet Gynecol 2008;112:1419-1444
- Cox JT, Schiffman M, Solomon D. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. ASCUS-LSIL Triage Study (ALTS) Group. Am J Obstet Gynecol 2003;188:1406-1412. (Level II-2)
- Schiffman M, Wentzensen N. From human papillomavirus to cervical cancer. Obstet Gynecol 2010;116:177-185
- Schiffman M, Rodriguez AC. Heterogeneity in CIN3 diagnosis. Lancet Oncol 2008;9:404-406
- Ferris DG, Litaker M. Interobserver agreement for colposcopy quality control using digitalized colposcopic images during the ALTS trial. J Low Genit Tract Dis 2005;9:29-35
- Mousavi AS, Fakour R, Gilani MM, et al. A prospective study to evaluate the correlation between Reid colposcopic index impression and biopsy histology. J Low Genit Tract Dis 2007;11:147-150
- Alonso I, Torne A, Puig-Tintore LM, et al. High-risk cervical epithelial neoplasia grade 1 treated by loop electrosurgical excision: follow-up and value of HPV testing. Am J Obstet Gynecol 2007;197:359.e1-6
- Pretorius RG, Peterson P, Azizi F, et al. Subsequent risk and presentation of cervical intraepithelial neoplasia (CIN)3 or cancer after a colposcopic diagnosis of CIN1 or less. Am J Obstet Gynecol 2006;195:1260-1265
- Cushieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemol Biomarkers Prev 2008;17:2536-2545
- Mulvany NJ, Allen DG, Wilson SM. Diagnostic utility of p16INK4a: a reappraisal of its use in cervical biopsies. Pathology 2008;40:335-344
- Overmeer RM, Henken FE, Snijders PJ, et al. Association between dense CADM1 promoter methylation and reduced protein expression in high-grade CIN and cervical SCC. J Pathol 2008;215:388-397
- Cardenas-Turanzas M, Freeberg JA, et al. The clinical effectiveness of optical spectroscopy for the in vivo diagnosis of cervical intraepithelial neoplasia: where are we? Gyncol Oncol 2007;107(1Suppl1):S138-146
- Chase DM, Kalouyan M, DiSaia PJ. Colposcpy to evaluate abnormal cervical cytology in 2008. Am J Obstet Gynecol 2009;200:472-480
- Kerimer AR, Guido RS, Solomon D, et al. Human papillomavirus testing following loop electrosurgical excision procedure identifies women at risk for post-treatment cervical intraepithelial neoplasia grade 2 or 3 disease. Cancer Epidemiol Biomarkers Prev 2006;15:908-914. (Level II-2)
- Wright TC Jr, Massad LS, Dunton CJ, et al. 2006 Consensus Guidelines for the Management of Women with Abnormal Cervical Screening Tests. 2006 ASCCP-Sponsored Consensus Conference. J Low Genit Tract Dis 2007;11:201-222
- ACOG Committee Opinion. Evaluation and management of abnormal cervical cytology and histology in adolescents. Number 436; June 2009
- Massad LS, Collins YC, Meyer PM. Biopsy correlates of abnormal cervical cytology classified using the Bethesda system. Gynecol Oncol 2001;82:516-522. (Level II-3)
- Stoler MH, Schiffman M. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study (ALTS) Group. JAMA 2001;285:1500-1505. (Level III)
- Trimble CL, Piantadosi S, Gravitt P, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res 2005;11:4717-4723. (Level II-2)
- Tate DR, Anderson RJ. Recrudescence of cervical dysplasia among women who are infected with the human immunodeficiency virus: a case-control analysis. Am J Obstet Gynecol 2002;186:880-882. (Level II-2)
- American Society for Colposcopy and Cervical Pathology. Management of women with atypical squamous cells of undetermined significance (ASCUS). Hagerstown (MD): ASCCP; 2007. Available at: http://www.asccp.org/pdfs/consensus/algorithms_cyto_07.pdf Accessed 2 August 2010
- Huh WK. Human papillomavirus infection. Obstet Gynecol 2009;114:139-143
- Massad LS, Evans CT, Weber KM, et al. Changes in knowledge of cervical cancer prevention and human papillomavirus among women with human immunodeficiency virus. Obstet Gynecol 2010;116:941-947
Published: 30 August 2012
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


