Cervical Glandular Carcinomas: Early Detection & Prevention
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Widespread use of Papanicolaou test for the screening of cervical cancers has lead to a significant decline in overall incidence and mortality rates over the past three decades. When different histologic types of cervical cancer are considered and trends are reexamined, it becomes apparent that observed declines are reflective of squamous cell carcinomas predominately; the rates for adenocarcinomas continue to rise. This rise in incidence may be due to the greater difficulty in screening for glandular precursor lesions that often arise high within the endocervical canal. Effective cervical cancer prevention requires recognition and treatment of the precursors of invasive cancer and includes standardized terminology to report cervical test results. Cervical glandular cell abnormalities are being identified cytologically as well as histologically in increasing numbers. In 1979, Christopherson, based on a large population-based series, estimated 1:239 ratio of cervical adenocarcinoma in situ (AIS) to squamous cell carcinoma in situ (CIS). Since then, the incidence of adenocarcinomas of the cervix has been increasing in relationship to squamous cancers. Most likely, the preinvasive glandular abnormalities are also increasing.
The purpose of this document is to define strategies for early diagnosis and management of cervical adenocarcinomas. These strategies reflect new information concerning the natural history of cervical carcinogenesis and the performance of screening and diagnostic tests, and they take into account the cost and efficacy of various treatment and follow-up options. This document will describe staging criteria and treatment for cervical glandular carcinomas. For practical purposes, it will focus on the glandular cells and adenocarcinoma histologies only.
Abbreviations:
ACS - American Cancer Society
ASC - atypical squamous cells
AGC - atypical glandular cells
AIS - adenocarcinoma in situ
CIN - cervical intraepithelial neoplasia
CIS - squamous cell carcinoma in situ
FIGO - The International Federation of Gynecology and Obstetrics
HPV - human papillomavirus
HSIL - high-grade squamous intraepithelial lesions
LSIL - low-grade squamous intraepithelial lesions
SCC - squamous cell carcinoma
SIL - squamous intraepithelial lesion
Prevalence:
Cervical cancer is the second most cancer in women worldwide, with an estimated yearly incidence of 500,000 that result in 273,000 deaths (1). The American Cancer Society (ACS) estimated that 12,900 new cases of cervical cancer were diagnosed in the United States in 2001 and that 4,400 deaths from cervical cancer resulted. An estimated 11,150 women were diagnosed with cervical cancer in 2007. Although the incidence of and deaths from cervical cancer are obvious, the financial burden of the disease cannot be ignored. In the United States alone, an estimated $ 4-$ 6 billion is spent each year on the screening and treatment of precancerous lesions and cervical cancer. This figure includes costs that are attributed to screening, follow-up evaluation of abnormal test results, and the treatment of cervical cancer (2). Most cervical cancer cases involve squamous cell carcinoma (SCC); the next most common type of cervical cancer is cervical adenocarcinoma. In the United States from 1973-1976, SCC accounted for 87.6% of all cervical cancers, and adenocarcinoma accounted for 12.4%. Surveillance Epidemiology and End Results data from 2001-2004 revealed that 69.3% of histologically confirmed cases of cervical cancer were SCC and that 24.9% were attributed to adenocarcinoma.
The number of new SCC cases has declined over the past few decades; however, the number of new adenocarcinoma cases that are diagnosed each year has risen slowly. More recent data through 2000 separates trends extend in black and white women. This clearly indicates the need for up-to-date, comprehensive analyses to determine more recent trends in incidence and death. It is interesting to note that the highest increases in incidence of adenocarcinoma in situ (AIS) and invasive adenocarcinoma are observed in younger women <50 years old. (3). Adenocarcinoma in situ (AIS) frequently associated with cervical intraepithelial neoplasia (CIN). Most data would suggest that 50% or more of AIS are seen with CIN. Although the entire endocervical canal may be involved, >95% of AIS occur at the squamocolumnar junction. Several studies suggest that abnormal glandular elements are associated with HPV 18. The Papanicolaou test, which is the primary screening technique, demonstrates a significant higher degree of sensitivity for detecting SCC and its precursors than it does for detecting glandular dysplasias, which leads to the shift in the SCC: adenocarcinoma incidence ratio (4).
Human Papillomavirus (HPV) and Cervical Cancer:
Oncogenic human papillomavirus (HPV) infection is a necessary cause (99.7%) of cervical cancer. Worldwide, approximately 70% of all cervical cancer cases are attributable to HPV types 16 and 18. The next 2 most prevalent oncogenic types are 45 and 31, which together account for an additional 10% of all cervical cancer cases (5). It is important to note that there is a distinct difference in the distribution of oncogenic virus types in SCC compared with adenocarcinoma. Data indicate that, in HPV-DNA-positive SCC, oncogenic virus type, is observed in 50-60% of cases and oncogenic virus type 18 is observed in 10-20% of cases (6). In contrast, oncogenic virus type 18 plays a more prominent role in adenocarcinoma. Type 18 accounts for an estimated 40-60% of adenocarcinoma cases, and oncogenic virus type 16 is observed in 30-55% of adenocarcinoma cases. In total, oncogenic virus types 16 and 18 are responsible for as many as 92% of oncogenic virus-induced adenocarcinoma. Oncogenic virus types 45 and 31 account for an additional 7-9% of adenocarcinoma.
Natural history of cervical adenocarcinoma:
Adenocarcinoma in situ (AIS) is the recognizable precursor to invasive adenocarcinoma. Unlike SCC, earlier neoplastic precursors (low-grade or high-grade lesions) to AIS and adenocarcinoma are not well-characterized. The duration of progression from AIS to adenocarcinoma has been estimated to be 5-13 years (7). It has been postulated that oncogenic virus types infect reserve cells of the transformation zone that are committed to glandular differentiation, which eventually leads to the proliferation of atypical glandular cells (AGC) and AIS. The primary screening methods that is used, the Papanicolaou test, is not as sensitive as liquid-based cytologic screening at the detection of AGC, AIS or adenocarcinoma. AIS is considered difficult to visualize colposcopically because these neoplasias are often higher in the endocervical canal or deeper in the tissue, which indicates a strong need for tissue sampling and histologic evaluation. The American Society for Colposcopy and Cervical Pathology recommends that all women with a diagnosis of AGC, of any subcategory, or AIS be evaluated with colposcopy and endocervical sampling. If HPV-DNA testing was not done with the initial screening, it should be completed when the colposcopy is performed. Additionally, women >35 years old should have endometrial sampling (8). If the biopsy does not detect neoplasia, HPV-DNA-positive women should have follow-up repeat cytologic evaluation with an HPV-DNA test at 6 months; if they were initially HPV-DNA-negative, the same follow up evaluation should be performed at 12 month.
Pathogenesis of cervical adenocarcinomas
The 2001 Bethesda System for abnormal glandular cytologic screening (9):
- Atypical glandular cells (AGC): specify endocervical, endometrial, or not otherwise specified
- Atypical glandular cells (AGC), favor neoplastic: specify endocervical, endometrial, or not otherwise specified
- Endocervical adenocarcinoma in situ (AIS)
- Adenocarcinoma
Cervical cytology reported as AGC or AIS, how should the patient be treated?
The 2001 Bethesda System categories were defined with the intention of providing additional information about the patient's risk of underlying high-grade dysplasia. In a review of 1,869 AGC results with histologic correlation, 33.7% were found to harbor SIL, 2.5% AIS, and 1% cervical adenocarcinoma, indicating that the most common significant lesions associated with AGC are actually squamous. The risk of CIN 2/3+ in women with AGC cytology results is 9-41%, in contrast to 27-96% with AGC favor neoplasia. At least half of AIS and adenocarcinoma results will be accompanied by squamous CIN. An AIS cytology result is associated with a 48-69% risk of histologic AIS and a 38% risk of invasive adenocarcinoma of the cervix (10). The balance between adequate disease detection and overly aggressive evaluation in women with AGC results in particularly challenging. Endocervical curettage and colposcopy are both relatively insensitive for AIS and adenocarcinoma, but most women with AGC cytology results do not have significant lesions.
The initial evaluation of women with AGC results is dictated by the risk of CIN 2/3+ noted previously, by the possibility that the source of the abnormality may be the endometrium, and by the recognition that the entire endocervix is at risk for AIS, mandating endocervical sampling. As a consequence, colposcopy and endocervical sampling should be included in the initial evaluation of all women with AGC results, except for those with results that specify "atypical endometrial cells". Women with atypical endometrial cells and a normal endometrial sampling should undergo colposcopy and endocervical sampling. Endometrial sampling is indicated in women with atypical endometrial cells and all women with AGC results who are aged 35 years or older, as well as those younger than 35 years with abnormal bleeding, morbid obesity, oligomenorrhea, or clinical evaluation suggesting endometrial cancer.
Initial evaluation of AGC or AIS do not reveal intraepithelial lesion or caner, how should the patient be treated?
Treatment of women with AGC and negative initial evaluations is determined by the risk that significant disease is present but was not detected. The category AGC - Not otherwise specific (NOS) is associated with a sufficiently low risk of missed disease that follow-up with repeat cytology testing and endocervical sampling four times at 6-month intervals is recommended. Although untested in a clinical trial, this prolonged follow-up scheme is recommended because of the recognized insensitivity of cytology testing and endocervical sampling for glandular neoplasias. Like squamous CIN, HPV is found in more than 95% of AIS and 90-100% of invasive adenocarcinomas of the cervix. The largest published series of AGC results uniformly evaluated with cervical histology and HPV testing found that 40 of 137 women (29%) were HPV positive, including 11 of 12 women with CIN 2 or CIN 3 and all 5 women with AIS (11). Similar reports suggest that it is reasonable to monitor women with AGC cytology test results, a negative initial evaluation, and a negative HPV test result with a repeat cytology and endocervical sampling in 1 year rather than requiring four visits at 6-month intervals. For women with reports of 1) AGC favor neoplasia or AIS cytology result and a negative initial evaluation, or 2) a second AGC-NOS result and a second negative evaluation, the risk of missing a significant lesion is sufficient that an excision is warranted. Cold knife conization is a good choice in this situation because of the prognostic importance in AIS of the pathologic evaluation of margins, which may be obscured by thermal artifact in some LEEP specimens. The rarity of this diagnosis and the difficulty with management may require consultation with expertise in this area, such as gynecologic-oncologist.
How should AIS be managed and monitored?
For the patient with a colposcopic biopsy diagnosis of AIS, exclusion of invasive cancer and the removal of all affected tissue is the primary goal. Hysterectomy is not appropriate until invasive cancer has been excluded. Excision is required to accomplish these goals. Cold-knife conization is recommended to preserve specimen orientation and permit optimal interpretation of histology and margin status, which are important to inform decisions about the risks of conservative management. Use of LEEP is this situation is associated with an increase in positive cone margins over cold-knife conization and is not recommended (12). Endocervical sampling immediately after conization has been reported to have better positive and negative predictor value for residual disease than cone margins. If the margins of the cone specimen are involved, conization should be repeated. The risk of residual AIS at subsequent conization or hysterectomy has been reported as high as 80% in patients with positive margins. , and most of the women found to have undiagnosed invasive cancer at hysterectomy are this group. Repeat conization is preferred to immediate hysterectomy so that if invasive cancer is found, the appropriate surgical or radiotherapeutic treatment can be recommended. If the margins of the cone specimen are not involved, risk of residual disease is still substantial. This is of concern because both cytology and endocervical curettage have substantial false-negative rates of glandular lesions, and there are multiple reports of the diagnosis of invasive adenocarcinoma after long periods of regular follow-up with negative screening results. This level of risk and insensitivity of screening tests supports a recommendation for hysterectomy when fertility is no longer desired.
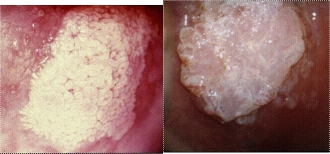
Adenocarcinoma in situ (AIS)
When fertility is desired and cervical conization margins are clear, conservative follow-up may be undertaken with cytology tests and endocervical sampling every 6 months, provided that the patient understands the risk of subsequent recognition or development of invasive cancer. Recognizing that the vast majority of AIS and invasive adenocarcinomas are HPV positive, some experts use the combination of HPV testing and cytology to further stratify risk and assist women with decisions regarding continued fertility (13). In pregnancy, the only diagnosis that may alter management is invasive cancer. Excisions should be considered for pregnant women only if a lesion detected at colposcopy is suggestive of invasive cancer.
Should squamous cell cancer and adenocarcinoma be treated differently?
Despite the ongoing discussion regarding cell type and prognosis, there is no evidence to support differences in treatment of invasive squamous cell cancer versus adenocarcinoma of the cervix. The only exception to this is the management of patients with FIGO stage Ia1 squamous cell cancer, corresponding with the Society of Gynecologic Oncologists' working definition for minimally invasive tumors discussed previously. It should be emphasized that no definition for microinvasive adenocarcinoma of the cervix has been agreed upon; therefore, treatment algorithms for such patients remain undefined (14). For patients with frankly invasive disease, regardless of squamous cell or adenocarcinoma histology, the primary options for treatment are radical hysterectomy with lymphadenectomy or definitive radiation therapy. In patients undergoing primary surgery who have positive nodes, positive margins, or parametrial infiltration, adjuvant radiation concurrent with cisplatin-based chemotherapy is indicated on the basis of positive results from a randomized trial. For more advanced disease in which primary radiation therapy is recommended, the addition of concurrent cisplatin-based chemotherapy provides clear therapeutic benefit.
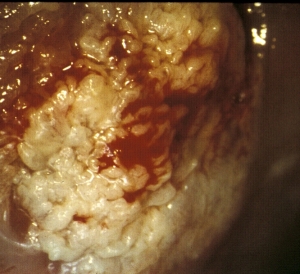
Cervical Cancer - Grossly squamous cell carcinoma and adenocarcinoma have similar appearances
Prevention:
Healthcare in the United States has become a privilege rather than a right. Patients who have the greatest need are the ones most likely to be denied this privilege. Despite recent advances in disease detection and treatment, many patients do not receive even the bare minimum of care. The high complexity of the health care system in the setting of patients with low levels of health literacy significantly affects the ability to seek and receive treatment in a timely fashion. In addition, lack of insurance, transportation, and social support further complicate access to care. To truly provide a standard of care to all patients, regardless of resources, the healthcare system in USA must evolve to address the needs of the population (15). Although the aforementioned techniques may detect adenocarcinoma at an earlier stage, they are not primary means to prevent adenocarcinoma. In this context, vaccination to prevent infection with oncogenic virus types is a primary means to prevent a significant portion of adenocarcinoma. A vaccine with 100% efficacy against oncogenic virus types 16 and 18 (the 2 most prevalent, causal HPV types that are detected in adenocarcinoma) could reduce the incidence of adenocarcinoma dramatically by preventing as many as 92% of cases. With regards to adenocarcinoma, it is important to consider end points concerning oncogenic virus type 18, which the type that is responsible for a greater proportion of adenocarcinoma compared with SCC. Both vaccines (quadrivalent L1 VLP 6/11/16/18 and bivalent L1 VLP 16/18) display outstanding efficacy against persistent infection and the development of cervical intraepithelial neoplasia 2+ lesion that are related to oncogenic types 16 and 18. The safety profiles for both vaccines are well-tolerated and no serious side effects have been reported (16).
Summary:
Reducing the incidence and mortality rates that are associated with adenocarcinomas can be accomplished by using improved screening techniques and large-scale implementation of cervical cancer vaccines that target the predominant oncogenic human papillomavirus types that are associated with adenocarcinoma. Cervical adenocarcinoma incidence and mortality rates continue to rise, despite large-scale implementation of cytologic screening in the United States. This is partly due to the limitations of current screening techniques to detect precursors to adenocarcinoma, which include AGC and AIS. As a result, women often are diagnosed at late stages of the disease, which results in poorer diagnosis. Endocervical sampling using a brush or curette may be undertaken as part of the evaluation of ASC and LSIL cytology results and should be considered as part of the evaluation of AGC, AIS and HSIL cytology results. Endocervical sampling is recommended at the time of an unsatisfactory colposcopy or if ablative treatment is recommended. Endocervical sampling is not indicated in pregnancy. Endometrial sampling is indicated in women with atypical endometrial cells and in all women aged 35 years or older who have AGC cytology results, as well as in women younger than 35 years with abnormal bleeding, morbid obesity, oligomenorrhea, or clinical results suggesting endometrial cancer.
Women with AGC favor neoplasia or AIS cytology results and negative or unsatisfactory colposcopy results should undergo excision unless they are pregnant. A colposcopic examination negative for abnormalities after two AGC-NOS cytology results is also an indication for excision in the absence of pregnancy. Women with a cervical biopsy diagnosis of AIS should undergo excision to exclude invasive cancer. Cold-knife conization is recommended to preserve specimen orientation and permit optimal interpretation of histology and margin status. After treatment of AIS, when future fertility is desired and cervical conization margins are clear, conservative follow-up may be undertaken with cytology and endocervical sampling every 6 months. Patients with squamous cell cancers and those with adenocarcinomas should be managed similarly, except for those with microinvasive disease. Criteria for microinvasive adenocarcinoma have not been established.
Acknowledgment: Women's Health and Education Center (WHEC) expresses gratitude to Dr. Bradley J. Monk, Associate Professor and Director of Research, Division of Gynecology Oncology, University of California Irvine Medical Center, California (USA) for his priceless assistance in developing the manuscript. We are looking forward to collaborate with him for many years to come. This information is designed to aid healthcare providers in making decisions about appropriate care of obstetric and gynecologic care. Variations in practice may be warranted based on the needs of the individual patient, resources, and limitations unique to the institution or type of practice.
References:
- Sherman ME, Wang SS, Carreon J, et al. Mortality trends for cervical squamous and adenocarcinoma in the United States: relation to incidence and survival. Cancer 2005;103:1258-1264
- Sankaranarayann R, Ferlay J. Worldwide burden of gynecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynecol 2006;20:207-225
- Wang SS, Sherman ME, Hildesheim A, et al. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976-2000. Cancer 2004;100:1035-1044
- Krane JF, Granter SR, Trask CE, et al. Papanicolaou smear sensitivity for the detection of adenocarcinoma of the cervix: a study of 49 cases. Cancer 2001;93:8-15
- Centers for Disease Control and Prevention. Genital HPV - CDC Fact Sheet, 2004
- Castellsague X, Diaz M, de Sanjose S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst 2006;98:303-315
- Lee KR, Flynn CE. Early invasive adenocarcinoma of the cervix. Cancer 2000;89:1048-1055
- Wright TC Jr, Massd LS, Dunton CJ, et al. 2006 Consensus guidelines for the management of women with abnormal cervical cancer screening test. Am J Obstet Gynecol 2007 [in press]
- Herzog TJ, Monk BJ. Reducing the burden of glandular carcinomas of the uterine cervix. Am J Obstet Gynecol 2007;197:566-571
- Raab SS. Can glandular lesions be diagnosed in Pap smear cytology? Diag Cytopathol 2000;23:127-133 (Level III)
- Krane JF, Lee KR, Sun D, et al. Atypical glandular cells of undermined significance: outcome prediction based on human papillomavirus testing. Am J Clin Pathol 2004;121:87-92. (Level III)
- Kennedy AW, Biscotti CV. Further study of the management of cervical adenocarcinoma in situ. Gynecol Oncol 2002;86:361-364
- ACOG Practice Bulletin. Management of abnormal cervical cytology and histology. Number 66, September 2005
- ACOG Practice Bulletin. Diagnosis and treatment of cervical carcinomas. Number 35, May 2002
- Westin SN, Bustillos D, Gano JB, et al. Social factors affecting treatment of cervical cancer - ethical issues and policy implications. Obstet Gynecol 2008;111:747-751
- Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006;95:1459-1466
Published: 24 June 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


