Benign Vulvar Skin Disorders: Part 2
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Vulvar skin disorders include a variety of inflammatory conditions of the vulva that also may affect the extragenital area. Pruritis and pain are two of the most common presenting symptoms in vulvar clinics. Vulvovaginal symptoms often are chronic and can adversely affect sexual function and sense of well-being. The skin functions as a physiologic barrier and a major organ of homeostasis. The practicing obstetrician and gynecologist can play an important role in identifying skin diseases and initiating management. Additionally, the skin often reflects internal disease states. An astute healthcare provider can identify systemic conditions early, with the goal of improving management.
The purpose of this document is to provide updated diagnosis and management of the genital ulcers. The following non-infectious vulvar ulcers: vulvar aphthae in adult and pediatric patients, aphthae associated with Behҫhet's disease, vulvar ulcers resulting from Crohn's disease are discussed. Genital ulcers because of common sexually transmitted diseases discussed are herpes simplex, syphilis, chancroid, lymphogranuloma venereum (LGV), granuloma inguinale (Donovanosis), vulvar ulcers associated with human immunodeficiency virus (HIV) infection.
A. Sexually Transmitted Diseases (STDs) Characterized by Genital Ulcers
The most common infectious etiologies of genital ulcer disease in the United States, in descending order of incidence are herpes simplex virus (HSV), syphilis, and chancroid. Less frequent causes of genital ulcers in the United States are HIV infection, LGV and granuloma inguinale.
I. Genital Herpes
Herpes genitalis is caused by the herpes simplex virus type 1 (HSV-1) or type 2 (HSV-2) and can manifest as primary or recurrent infection. It is one of the most common sexually transmitted infections and due to associated physical and psychological morbidity it constitutes a considerably, often underestimated medical problem. Both organism, HSV-1 and HSV-2 are enveloped DNA viruses that are sensitive to disinfectants and environmental factors (1). Due to marked genetic homology between HSV-1 and HSV-2 numerous biological similarities and antigenic cross- reactions between viruses exist. Type-specific epitopes include the viral glycoprotein (g) gG (HSV-1 and HSV-2) and gC (HSV-1) (1).
Mode of transmission: the primary mode of both HSV-1 and HSV-2 is through direct contact. Genital HSV-2 infection is associated with an increased risk of HIV infection (2). Primary genital infections with HSV-1 and HSV-2 are usually asymptomatic. The classical clinical features consist of macular or popular skin and mucous membrane lesions occurring approximately 4-7 days after sexual contact; these progress to vesicles, pustules and ulcers. It can last for up to 3 weeks. Typical symptoms also include pain, especially painful inflammatory swelling of the vulva in women, burning pain and dysuria. Lymphadenopathy, fever and cervicitis (in women) / proctitis (in men) are relatively common associated symptoms. Genital herpes may manifest atypically, particularly in the female genital tract, such as a solitary lesion and fever, making the clinical diagnosis far more difficult. Signs of herpes lesions of the cervix are relatively common in the absence of symptoms, while urethral manifestations are often associated with severe micturition problems.
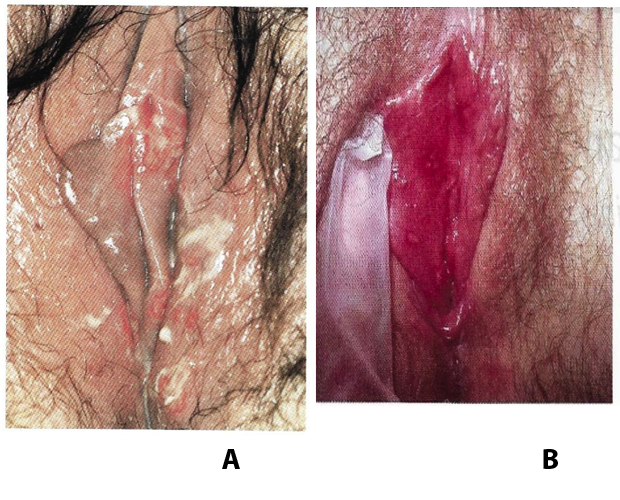
Figure 1. A: Herpes vulvitis, multiple genital ulcerations. B: a solitary herpes simplex vulvar lesion.
Recurrent herpes genitalis follows the primary eruption the virus establishes lifelong latency in sensory neural ganglions; in the case of primary genital infection the sacral ganglions are mainly involved. From here the virus can reactivate, causing recurrent infection. Recurrences occur in almost every person suffering symptomatic primary herpes genitalis due to HSV-2, in a third of patients frequently (at least 6 times a year). Recurrent HSV-1 infections occur over 5 times less commonly (3).
Asymptomatic genital viral shedding: In the majority of cases endogenous viral reactivation is characterized by asymptomatic genital viral shedding. Most commonly HSV-2 is shed by HSV-2 seropositive patients, and this is the case for almost anyone who is anti-HSV-2 IgG positive (4). In contrast, HSV-1 shedding is uncommon. These data allow the assumption, with a high level of certainty, that HSV-2 seropositive people should always be regarded as potential virus excretors.
Both primary and recurrent HSV infection in pregnant women can result in intrauterine viral transmission and congenital HSV infection, although the incidence is low at just 5% of all HSV infections in newborns (5). Summary of virology and serology findings for the laboratory diagnosis of HSV infections with or without genital lesions and for asymptomatic viral shedding is provided in the table 1 below (6).
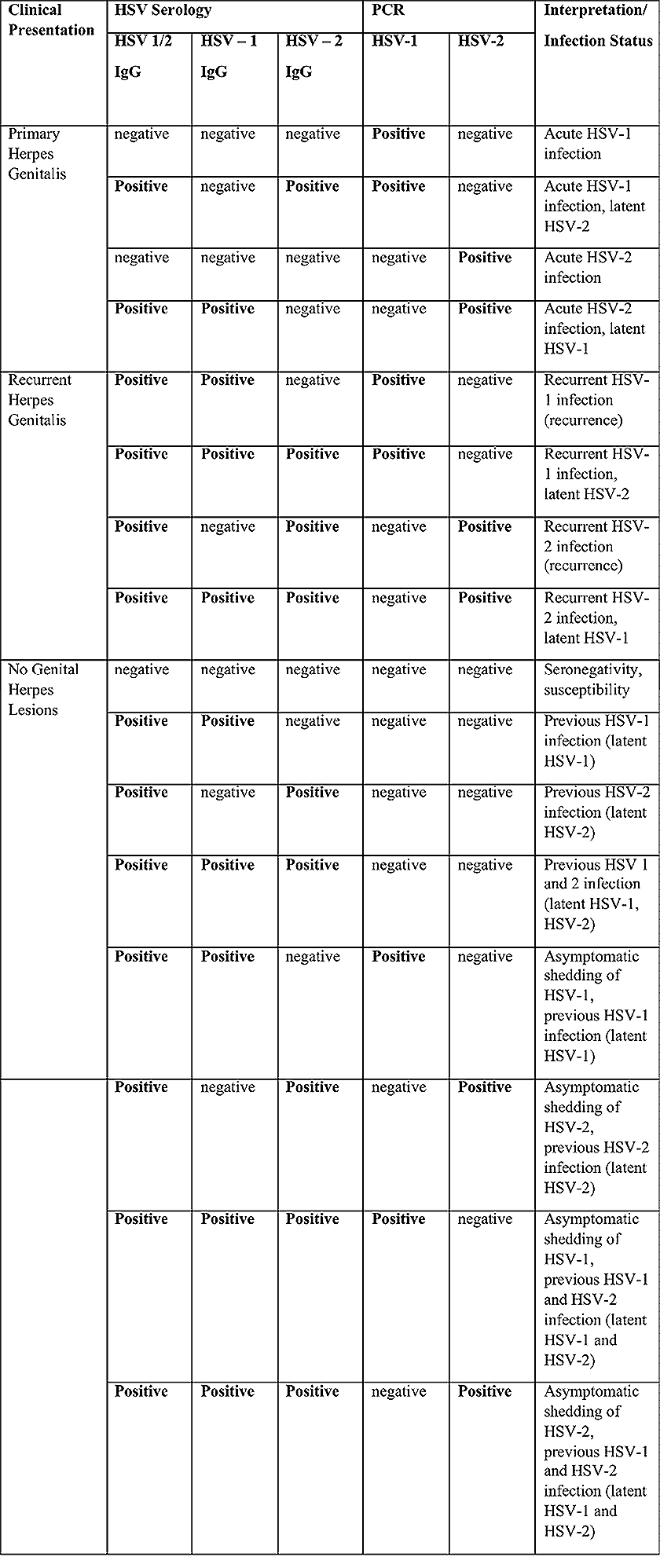
Table 1. A: HSV Laboratory findings and correlating herpes genitalis symptoms. Abbreviations: HSV (herpes simplex virus); PCR (polymerase chain reaction); IgG (viral glycoprotein g)
Summary of the recommended viral diagnostic algorithm for herpes genitalis
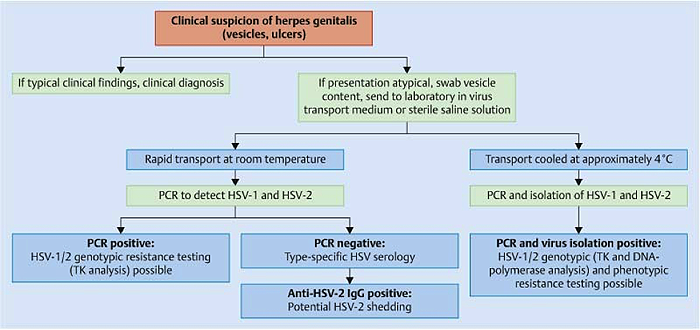
Figure 2. A: Viral diagnostic algorithm for herpes genitalis. Abbreviation: TK – thymidine kinase; PCR – polymerase chain reaction
Antiviral Therapy
Standard first-line drugs include acyclovir, valacyclovir and famciclovir. The specific antiviral action of these acyclic nucleoside analogues is based on their phosphorylation to monophosphate form by thymidine kinase (TK), the key enzyme of HSV-1 and HSV-2, with subsequent phosphorylation via di- to tri-phosphate form by cellular enzymes (7).
Primary herpes genitalis
Acyclovir:
- 3 x 400 mg by mouth daily, for 7 - 10 days;
- or 5 x 200 mg by mouth daily, for 7-10 days.
Valacyclovir: 2 x 500 mg by mouth daily, for 7 - 10 days.
Famciclovir: 3 x 250 mg by mouth daily, for 7 - 10 days.
Severe primary herpes genitalis
Acyclovir: 3 x 5 mg/kg intravenous (I.V.) daily, for 5 - 7 days, 10 days for immunosuppressed.
Primary herpes genitalis during pregnancy
Acyclovir: 5 x 200 mg by mouth daily, for 10 days
Recurrent herpes genitalis (5 - 6 episodes per year)
Acyclovir:
- 2 x 800 mg by mouth daily, for 5 days; or
- 3 x 400 mg by mouth daily, for 5 days; or
- 3 x 800 mg by mouth daily, for 2 days.
Valacyclovir:
- 2 x 500 mg by mouth daily, for 3 days; or
- 1 x 1,000 mg by mouth daily, for 5 days
Famciclovir:
- 2 x 125 mg by mouth daily, for 5 days; or
- 2 x 1,000 mg by mouth daily, for 1 day.
Mild presentations can also be treated topically with acyclovir or foscarnet. This not adequate during pregnancy. In immunocompromised patients (e.g. HIV) higher doses, longer treatment periods and acyclovir IV may be necessary.
Recurrent herpes genitalis during pregnancy
Acyclovir: 3 x 400 mg by mouth daily, for 10 days; preventive: from 36th gestational week to delivery. Note: acyclovir is not licensed for use during pregnancy (off-label use). Use should be avoided particularly before 15th week of gestation.
Valacyclovir: 2 x 250 mg by mouth daily, for 3 days; preventive: 36th gestational week to delivery.
Preventive treatment before pregnancy
Acyclovir: 2 x 400 mg by mouth daily, maximum 6 months.
Prophylaxis / viral suppression (>5 – 6 episodes per year)
Acyclovir: 2 x 400 mg daily by mouth, maximum 6 months; or
4 x 200 mg daily by mouth, maximum 6 months.
Valacyclovir: 1 x 500 mg by mouth daily, maximum 6 months.
Famciclovir: 2 x 250 mg by mouth daily, maximum 6 months.
According to a Cochrane study (8), viral suppression / prophylaxis may already be indicated after at least 4 recurrences per year. Longer than 6 months in certain settings (e.g. HIV). Prerequisite for viral suppression / prophylaxis: monthly renal and liver laboratory parameters.
Recurrent herpes genitalis with acyclovir
Foscarnet: 3 x 40 ( - 80) mg/kg intravenous (IV) daily, until clinical improvement (maximum 20 days). Alternative: Cidofovir 5 mg/kg IV 1 x weekly, later 2 x weekly, (off-label use); or 1% foscarnet cream or 1% cidofovir gel topically.
II. Syphilis
Treponema pallidum subspecies pallidum (T. pallidum) causes syphilis via sexual exposure or vertical transmission during pregnancy. The spirochete has a long latent period during which patients have no signs or symptoms but can remain infectious.
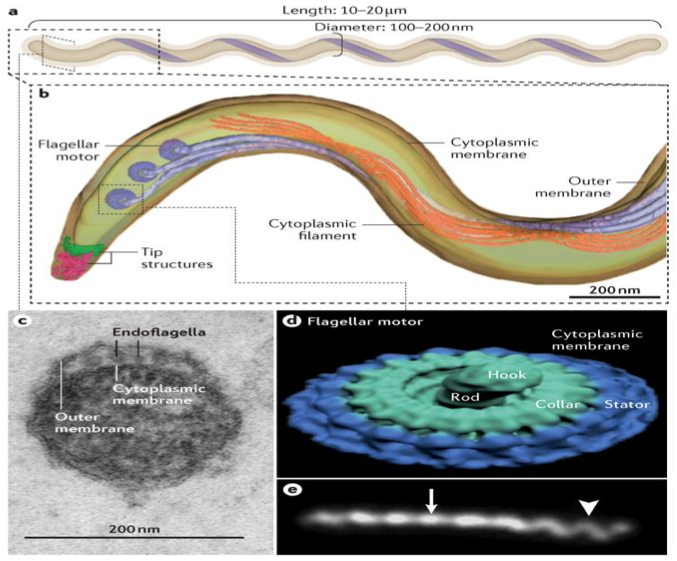
Figure 3. a: T. pallidum consists of a protoplasmic cylinder and cytoplasmic membrane bounded by thin peptidoglycan sacculus and outer membrane. b: periplasmic flagellar filaments, a defining morphological feature of spirochetes, originate from nanomotors situated at each pole and wind around the cylinder atop the peptidoglycan deforms the sacculus to create the flat wave morphology of the spirochete. c: ultra-thin section of T. pallidum showing the outer and cytoplasmic membranes and flagellar filaments (endoflaggella) within the periplasmic space. d: surface rendering of a flagellar motor based on cryoelectron tomograms. e: darkfield micrograph showing the flat-wave morphology of T. pallidum. The arrow and arrowhead indicate segments that are oriented 90 deg. from each other.
Transmission and Dissemination
Transmission of venereal syphilis occurs during sexual contact with an actively infected partner; exudate containing as few as 10 organisms can transmit disease (9). Spirochetes directly penetrate mucous membranes or enter through abrasions in skin, which is less heavily keratinized in peri-genital and peri-anal areas than skin elsewhere. Once below the epithelium, organisms multiply and begin to disseminate through the lymphatics and bloodstream. The infection rapidly becomes systemic, if untreated. Profuse spirochetes within the epidermis and superficial dermis in secondary syphilitic lesions enable tiny abrasions created during sexual activity to transmit infection (10). Penetration of the blood-brain barrier, occurring in as many as 40% of individuals with untreated syphilis, can cause devastating neurological complications (11). Neurosyphilis is typically described as a late manifestation but can occur in early syphilis. Symptomatic manifestations of neurosyphilis include chronic meningitis, meningovascular stroke like syndromes and manifestations common in the neurological forms of tertiary syphilis (namely, tabes dorsalis and general paresis, a progressive dementia mimicking a variety of psychotic syndromes) (11).
Diagnosis, screening and prevention
A syphilis diagnosis is often based on a suggestive clinical history and supportive laboratory (that is, sero-diagnostic) tests. The classically painless lesions of primary syphilis can be seen on the sites of exposure. Sometimes can be missed, especially in hidden sites such as the cervix and rectum.
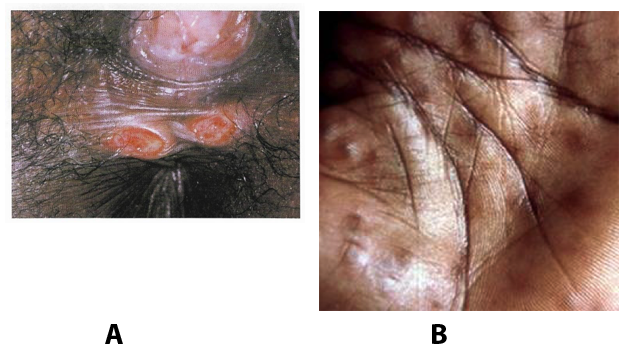
Figure 4. A: Primary syphilis genital ulcers. B: Secondary syphilis as palmar rash
Serological testing has become the most common means to diagnose syphilis whether in people with symptoms of syphilis, or in those who have no symptoms but are detected through screening. A limitation of all syphilis serological tests is their inability to distinguish between infection with T. pallidum subsp. Pallidum and the non-venereal T. pallidum subspecies that cause yaws, pinta or bejel.
Definitive Diagnosis by Direct Detection: The choice of method for diagnosing syphilis depends on the stage of disease and the clinical presentation. In patients presenting with primary syphilitic ulcers, condyloma lata (genital lesions of secondary syphilis) or lesions of congenital syphilis, direct detection methods – which include darkfield microscopy, fluorescent antibody staining, immunohistochemistry and PCR (polymerase chain reaction), may be used to make a microbiological diagnosis. However, with the exception of PCR, these methods are insensitive and require fresh lesions from which swab or biopsy material can be collected as well as well-experienced technologists. T. pallidum PCR is a developing technology that is still primarily available in research laboratories, although these tests are anticipated to be more widely available in the near future. Recent research indicates that this technology may be helpful for the diagnosis of neurosyphilis by the detection of T. pallidum DNA in the cerebrospinal fluid (CSF) of patients with syphilis, particularly among HIV-infected individuals (13).
Global Estimates of Prevalence
According to most recent estimation of the World Health Organization (WHO), approximately 17.7 million individuals 15 - 49 years of age globally had syphilis in 2012, with an estimated 5.6 million new cases every year (13). Syphilis testing and treatment during pregnancy is highly effective and was included in the Lives Saved Tools of effective maternal-child health intervention (14)
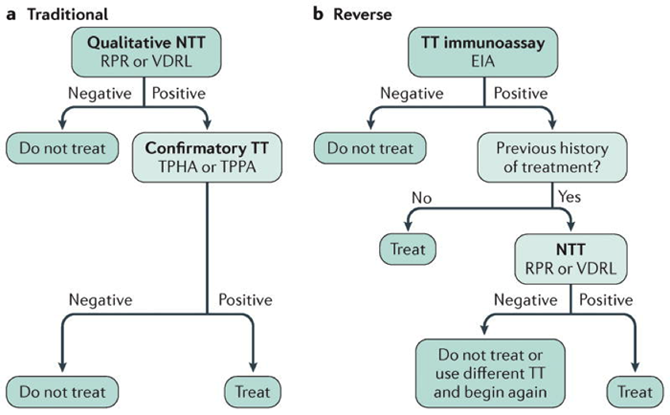
Figure 5. Screening algorithms for syphilis. Abbreviations: NTT (non-treponemal test); TT (treponemal test); EIA (enzyme immunoassay); RPR (rapid plasma regain); VDRL (venereal disease research laboratory); TPHA (T. pallidum hemagglutination assays); TPPA (T. pallidum passive particle agglutination assay.
WHO Guidelines for the Treatment of Syphilis (15):
Early syphilis
- Intramuscular (IM) benzathine penicillin G 2.4 million units (single dose); or
- IM procaine penicillin 1.2 million units (daily doses for 10 – 14 days); or
- If penicillin-based treatment cannot be used, oral doxycycline 100 mg twice daily orally for 14 days or ceftriaxone 1 g IM once daily for 10 - 14 days, or in special circumstances, azithromycin 2 g once orally.
Remarks: Doxycycline is preferred over ceftriaxone due to its lower cost and oral administration. Doxycycline should not be used in pregnant women. Azithromycin is on option in special circumstances only when local susceptibility to azithromycin is likely. If the stage of syphilis is unknown, recommendations for people with late syphilis should be followed.
Late syphilis
- IM benzathine penicillin G 2.4 million units once weekly for 3 consecutive weeks; or
- IM procaine penicillin 1.2 million units once daily for 20 days
- If penicillin-based treatment cannot be used, oral doxycycline 100 mg twice daily orally for 30 days.
Remarks: the interval between consecutive doses of benzathine penicillin should not exceed 14 days. Doxycycline should not be used in pregnant women.
Congenital syphilis
- Intravenous (IV) aqueous benzyl penicillin 100,000 to 150,000 U/kg/day for 10 - 15 days; or
- Procaine penicillin 50,000 U/kg/day single dose IM for 10 - 15 days
Remarks: if an experienced venipuncturist is available, aqueous benzyl penicillin may be preferred instead of IM injections of procaine penicillin.
Pregnant Women (early syphilis)
- Benzathine penicillin G 2.4 million units once IM; or
- Procaine penicillin 1.2 million units IM daily for 10 days; or
- If penicillin cannot be used (e.g. due to penicillin allergy or not available), erythromycin 500 mg orally 4 times daily for 14 days or ceftriaxone 1 g IM once daily for 10 - 14 days or azithromycin 2 g once orally.
Remarks: although erythromycin and azithromycin treat the pregnant women, they do not cross the placental barrier completely and as a result the fetus is not treated. It is therefore necessary to treat the newborn infant soon after delivery. Ceftriaxone is an expensive option and is injectable. Doxycycline should NOT be used in pregnant women.
III. Chancroid
Chancroid is a sexually transmitted disease caused by the Gram-negative bacterium Haemophilus ducreyi. It is characterized by necrotizing genital ulceration which may be accompanied by inguinal lymphadenitis or bubo formation.
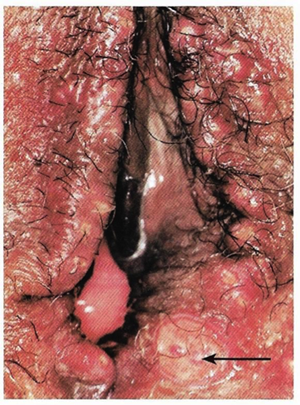
Figure 6. Chancroid. Arrow indicates the lesion.
H ducreyi is a fastidious organism which is difficult to culture from genital ulcer material. DNA amplification techniques have shown improved diagnostic sensitivity but are only performed in few laboratories (16). The management of chancroid in the tropics tends to be undertaken in the context of syndromic management of genital ulcer disease and treatment usually with erythromycin. A number of single dose regimens are also available to treat H ducreyi infection. Genital ulceration as a syndrome has been associated with increased transmission of HIV infection in several cross sectional and longitudinal studies. Effective and early treatment of genital ulceration is therefore an important part of any strategy to control the spread of HIV infection in tropical countries.
Recommended treatment regimens for chancroid from the WHO, CDC (Centers for Disease Control and Prevention) and the United Kingdom Clinical Effectiveness (CEG)
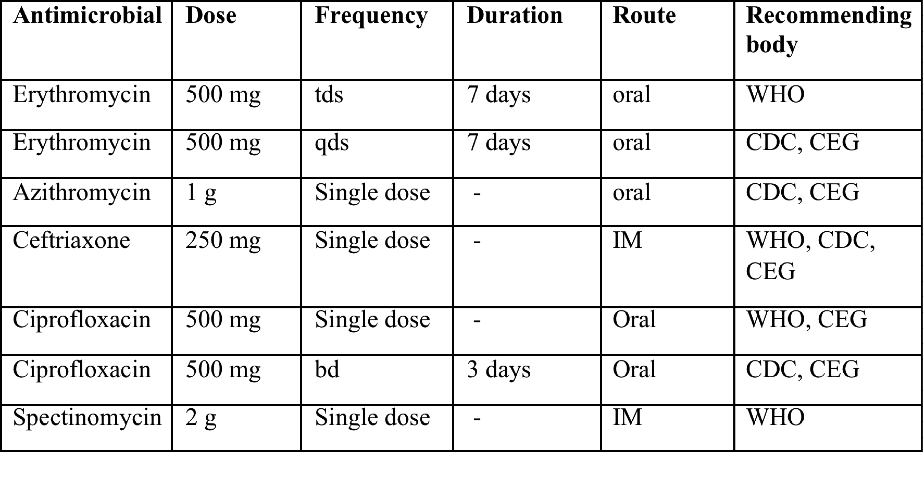
Table 2. A: bd = twice a day; tds = three times a day; qds = four times a day, IM - intramuscular.
Other Management Issues
Fluctuant buboes should be aspirated in order to provide symptomatic relief and to avoid the further complication of spontaneous rupture. Incision and drainage of fluctuant buboes, with subsequent packing of the wound, has also been recommended as an effective management strategy of chancroid and avoids the need for frequent bubo re-aspiration. In countries where the practice of syndromic management is adopted, patients with genital ulcers should receive treatment for both chancroid and syphilis. Therapy for granuloma inguinale should be added to the regimen in endemic areas and treatment for lymphogranuloma venereum should be given if inguinal buboes are present.
Patients with genital ulcers should be seen after treatment to ensure that healing has occurred, to exclude the possibility of reinfection, and to ensure that partner notification has taken place. All patients with genital ulcers should receive appropriate health education and safer sex practices should be discussed. Serological screening for both syphilis and HIV infection should be offered at the time of genital ulcer presentation and again after 3 months at the end of the window period for both diseases.
IV. Lymphogranuloma Venereum (LGV)
Lymphogranuloma venereum (LGV) is a sexually transmitted disease caused by L1, L2 and L3 serovars of Chlamydia trachomatis that primarily infects the lymphatics. In the last 10 years outbreaks have appeared in North America, Europe, and Australia in the form of proctitis among men who have sex with men. Three stages have been described:
First stage (primary LGV): The incubation period lasts 3 – 30 days, after which a primary lesion occurs in the form of a small papule, pustule, nodule, shallow erosion, or herpetiform ulcer. The initial lesions may be differentiated from the more common herpetic lesions by the lack of pain associated with the lesion. The primary lesion of LGV is most commonly located on the coronal sulcus of men and on the posterior vaginal wall, fourchette (known as the frenulum of labia minora/posterior commissure of labia minora) or vulva and on the cervix of women. The lesion usually heals within 1 week and may go unnoticed in the urethra, vagina, or rectum. Differentiation from a syphilitic chancre requires serologic testing.
Secondary lesions (secondary LGV): It begins within 2-6 weeks after the onset of primary lesion. Depending on the site of inoculation. LGV can cause inguinal syndrome (after primary lesion of the anterior vulva, penis, or urethra) or anorectal syndrome (usually after primary lesion of the posterior vulva, vagina, or anus). When both inguinal and femoral lymph nodes are involved, they can be separated by the inguinal (Poupart's) ligament, "Groove sign." This sign is pathognomonic of LGV but occurs in only 15% - 20% of cases (17). The systemic spread of LGV C. trachomatis may be associated with low-grade fever, chills, malaise, myalgias, and arthralgias. In addition, systemic spread occasionally results in arthritis, pneumonitis, abnormal hepatic enzymes, and perihepatitis. Rare systemic complications include cardiac involvement, aseptic meningitis, and ocular inflammatory disease. In the rare pharyngeal syndrome affecting the mouth and throat, cervical lymphadenopathy and buboes can occur.
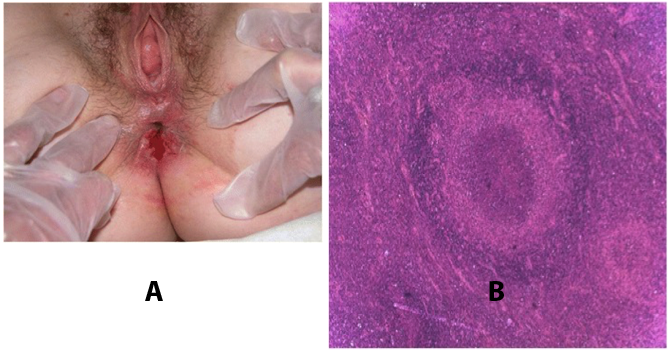
Figure 7. A: Ulcerative, painless lesion with lymphogranuloma venereum (LGV); B: Hematoxylin and eosin stained histological section of lymph node, with areas of necrosis and "starry abscesses".
Tertiary stage (third-stage LGV, genito-anorectal syndrome): This stage manifest predominantly in women, but also in homosexual men, because of the location of the involved lymphatics. It is characterized by a chronic inflammatory response and the destruction of tissue, which is followed by the formation of perirectal abscess, fistulas, strictures, and stenosis of rectum. Lymphorroids are hemorrhoids-like swellings of obstructed perirectal, and intestinal lymphatics may also occur. If it is not treated, chronic progressive lymphangitis leads to chronic edema and sclerosing fibrosis. This results in strictures and fistulas that can cause elephantiasis of the genitals, esthiomene (chronic ulcerative disease of vulva leading to disfiguring fibrosis and scarring), and frozen pelvis syndrome.
Diagnosis
Modern techniques now rely on nucleic acid amplification tests (NAATs) in well-equipped laboratories (18). The assays have high sensitivity and specificity. For the detection of LGV serovars of C. trachomatis different DNA samples can be used: 1) primary anogenital lesion swab (ulcer base exudate), 2) rectal mucosa swab (when anorectal LGV is suspected), or 3) enlarged or fluctuant lymph nodes or buboes aspirate (when inguinal LGV is suspected.
The second step diagnostic test is performed only if the first step test detects C. trachomatis in the sample. The second step diagnostic test in LGV biovar-specific DNA NAAT from the same sample used in the first step test. Two tests for the second step are available: a real-time PCR-based test and a real-time quadriplex PCR-based assay. Both PCR techniques are reliable; the assay correctly identified 100% of non-LGV chlamydial specimens, 100% with no chlamydial infection, and 96.36% LGV specimens (19). Histology of lymph nodes is not specific: follicular hyperplasia, abscesses, cryptitis and crypt disease abscesses without distortion of crypt architecture.
Management
Recommended treatment regimens for LGV (20)
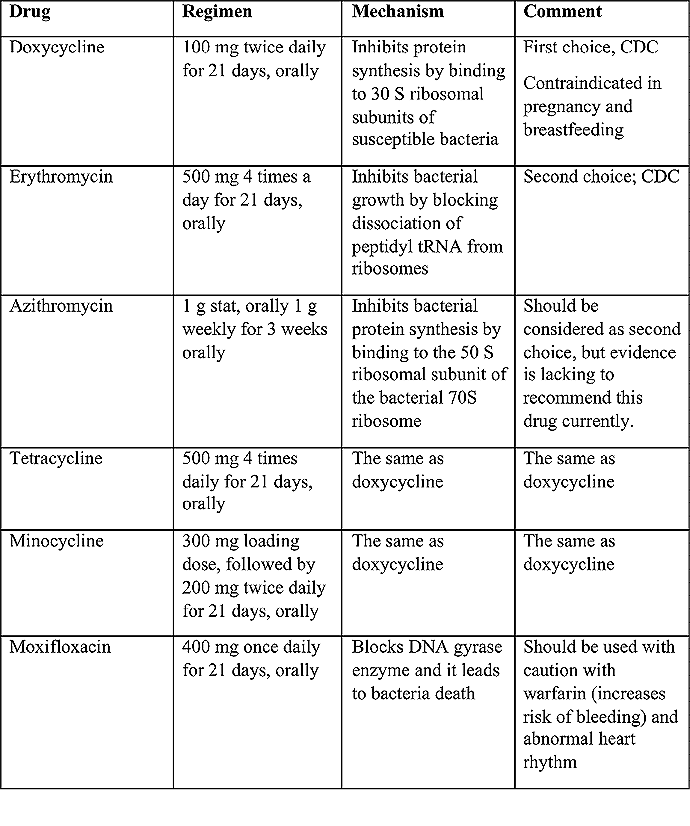
Table 3. A: Current guidelines in the United States by Centers of Disease Control and Prevention (CDC)
As adjunctive therapy, the aspiration of fluctuant buboes is recommended for pain relief and prevention of rupture or chronic sinus formation, in contrast to surgical incision of buboes due to potential complications. The pharynx is a reservoir for chlamydia and LGV and may play a role in ongoing transmission. Although spontaneous clearance may occur in untreated patients with pharyngeal chlamydia, in high-risk STD clinic patients, testing the pharynx for chlamydia should be considered.
V. Granuloma inguinale (Donovanosis)
Granuloma inguinale is a genital ulcerative disease caused by the intracellular gram-negative bacterium Klebsiella granulomatis. The disease occurs rarely in the United States, although it is endemic in some tropical and developing areas, including India, Papua, New Guinea, the Caribbean, central Australia, and southern Africa (21). Clinically, the disease is commonly characterized as painless, slowly progressive ulcerative lesions on the genitals or perineum without reginal lymphadenopathy; subcutaneous granulomas (pseudobuboes) also might occur. The lesions are highly vascular (i.e., beefy red appearance) and bleed. Extragenital infection can occur with extension of infection to the pelvis, or it can disseminate to intra-abdominal organs, bones, or the mouth. The lesions also develop secondary bacterial infection and can coexist with other sexually transmitted pathogens.
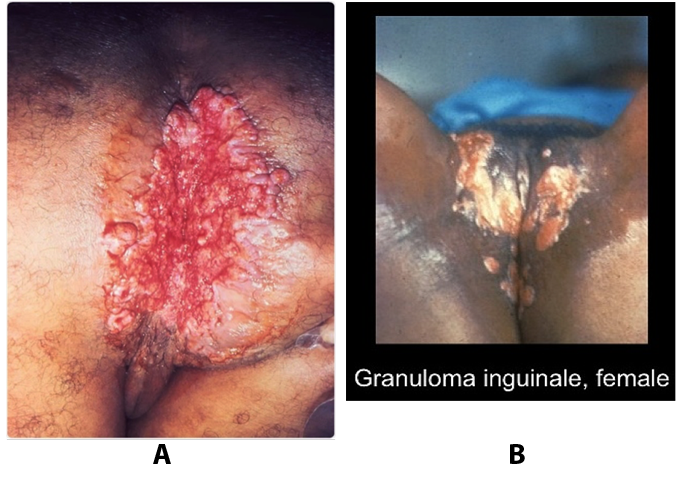
Figure 8. A: a very large erosive cutaneous lesion in the perianal region of the patient diagnosed with granuloma inguinale (Donovanosis). B: granuloma inguinale genital ulcers.
Diagnostic Considerations
The causative organism of granuloma inguinale is difficult to culture, and diagnosis requires visualization of dark-staining Donovan Bodies on tissue crush preparation or biopsy. No FDA-cleared molecular tests for the detection of K. granulomatis DNA exist, but such an assay might be useful when undertaken by laboratories that have conducted a CLIA verification study.
Tissue biopsy and Wright-Giemsa stain are used to aid in the diagnosis. The presence of Donovan bodies in the tissue sample confirms donovanosis. They were discovered by Charles Donovan. Donovan bodies are rod-shaped, oval organisms that can be seen in the cytoplasm of mononuclear phagocytes or histocytes in tissue samples from patients with granuloma inguinale.
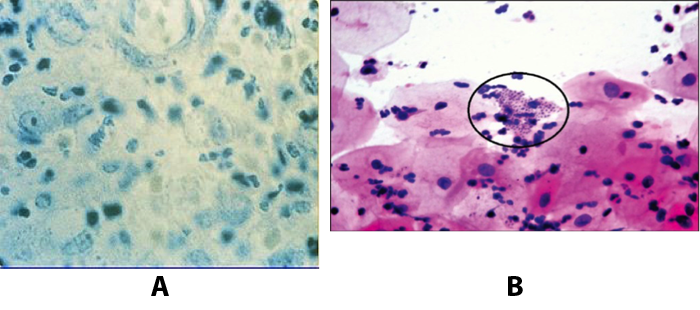
Figure 9. A: Donovan bodies rod shaped, oval organisms, intracellular inclusions in histocyte. B: Macrophages show numerous safety pin shaped structures with polar thickening of chromatin (Donovan bodies) and a halo around them in Pap smear (x100).
Treatment
Several antimicrobial regimens have been effective, but only a limited number of controlled trials have been published (21). Treatment has been shown to halt progression of lesions, and healing typically proceeds inward from the ulcer margins; prolonged therapy is usually required to permit granulation and re-epithelialization of the ulcers. Relapse can occur 6-18 months after apparently effective therapy.
Recommended Regimen (CDC):
Azithromycin 1 g orally once per week or 500 mg daily for at least 3 weeks and until all lesions have completely healed.
Alternative Regimens(CDC):
Doxycycline 100 mg orally twice a day for at least 3 weeks and until all lesions have completely healed; Or
Ciprofloxacin 750 mg orally twice a day for at least 3 weeks and until all lesions have completely healed; Or
Erythromycin base 500 mg orally 4 times a day for at least 3 weeks and until all lesions have completely healed; Or
Trimethoprim-sulfamethoxazole 1 double-strength (160 mg/800 mg) tablet orally twice a day for at least 3 weeks and until all lesions have completely healed.
The addition of another antibiotic to these regimens can be considered if improvement is not evident within the first few days of therapy. Addition of an aminoglycoside to these regimens is an option (gentamicin 1 mg/kg IV every 8 hours).
Other management considerations: Persons should be followed clinically until signs and symptoms have resolved. All persons who receive a diagnosis of granuloma inguinale should be tested for HIV and syphilis.
VI. HIV (Human Immunodeficiency Virus)-associated Vulvar Ulcer
Numerous observational studies have reported an association between genital ulcer disease (GUD) and an increased risk of HIV acquisition in HIV-negative individuals (22). It is plausible that, in and HIV-negative , GUD might increase susceptibility to HIV infection by disrupting the mucosal barrier and by inflammatory changes, which increase recruitment of HIV target cells to the ulcer. To assess the timing of symptomatic GUD relative to HIV seroconversion, in this study (22), concluded prevalence of GUS was increased among case subjects, at visit 2 and HIV load was increased in HSV-2 seropositive case subjects, compared with that in HSV-2 seronegative subjects, at 5 and 15 months after seroconversion. HIV acquisition is associated with HSV-2 seropositivity, and GUD is increased after seroconversion. HIV load is increased in HSV-2 positive subjects who seroconverted, suggesting a role for treatment of HSV-2 infection in HSV-2 infection in HSV-2 seropositive, dually infected individuals.
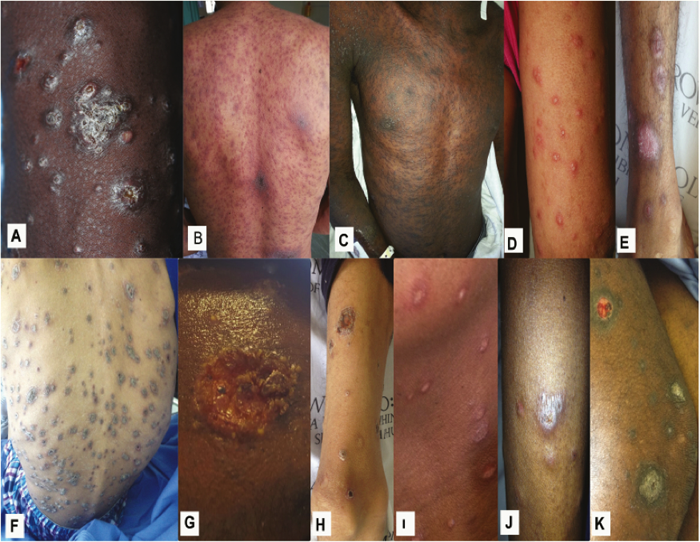
Figure 10. Selected cutaneous lesions with advanced HIV infection and generalized, recent-most skin lesions suspicious of systemic mycoses. Lesions shown are from patients with proven mycoses caused by Emergomyces africanus (A-E). Sporothrix schenckii (F-H), and Histoplasma capsulatum (I-K).
Symptomatic GUD is increased as a consequence of HIV acquisition, probably as a result of reactivation of pre-existing HSV-2 infection. Also, there is evidence that HSV-2 up-regulates HIV load and causes GUD, suggesting a role of treatment/prophylaxis of HSV-2 infection in individuals at risk for HIV and in newly HIV-infected individuals.
Most studies examining HIV-associated ulcers are conducted in Africa and involve the risk of transmission and co-infection with herpes simplex, syphilis, or chancroid. HIV-associated ulcers on vulva and genitals are similar to ulcers that affect the oral mucosa, esophagus, and rectum in these patients, and pathogenesis typically may be the same.
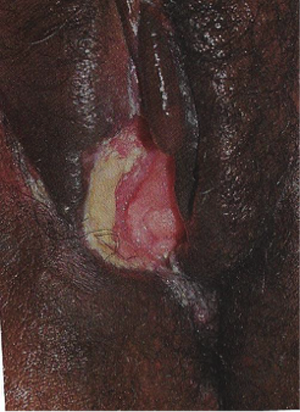
Figure 11. Large, destructive vulvar ulcer associated with HIV.
HIV-associated ulcers are typically large, painful, and recurrent. Patients are generally severely immunocompromised with a low CD4 count and AIDS-defining infections. The pathogenesis of HIV-associated ulcers remain unknown. Immunosuppression, altered host response and direct infection by HIV are proposed etiologies. In support of these theories is the fact that the CD4 count is typically low, and some women respond to antiretroviral therapy. Local destruction by non-healing ulcers can be severe, with formation of a recto-vaginal fistula reported and a labial-vaginal fistula extending to the ischiorectal fossa.
Although biopsies are usually non-diagnostic, obtaining one as part of evaluation is nevertheless recommended in patients with HIV-associated ulcer both to rule out malignancy and as a method of searching for potentially treatable infectious causes such as CMV, HSV, and mycobacterial infections. All women presenting with HIV-associated ulcers should additionally be screened for HSV and syphilis. Screening for chancroid, LGV and granuloma inguinale in select patients with high-risk behavior or from endemic regions is also prudent.
Early identification and treatment of HIV-associated vulvar ulcers are essential given the potentially crippling sequalae. Variably favorable response typically is seen with either topical, intralesional or oral corticosteroids (23). High-dose corticosteroids are reported to promote healing of individual patients, although the ideal dosing regimen has not yet been studied. Antiviral therapy with acyclovir is not beneficial (23). Given use of thalidomide for the treatment of complex aphthosis and aphthae of Behҫhet's disease (BD), this drug has been used to treat HIV-associated ulcers. The largest trial is a randomized controlled trial of treatment group received 200 mg/day of thalidomide. After 4 weeks of treatment, 55% of these patients had complete healing of ulcers compared with only 7% of the placebo group (23).
B. Non-Infectious Vulvar Ulcers
Ulcers should be differentiated from erosion both clinically and histologically. This is accomplished on the basis of depth. An erosion is a defect in the epidermis only, resulting in a red, smooth, moist, superficial, atrophic plaque. Ulcers are deeper, extending into the underlying dermis, and appear necrotic at the base with either yellow, fibrinous material or an eschar (a dry scab) adherent to it. Large, deep, and long-standing ulcers heal with scarring, while erosions and superficial ulcers typically heal without scarring. Non-infectious ulcers include those caused by drug reactions or adverse effects, autoimmune or inflammatory disease, trauma, and aphthous ulcers.
I. Aphthous Ulcers
Oral aphthae (aphthosis, aphthous stomatitis, recurrent aphthous ulcers) have similar characteristics to vulvar aphthae. Commonly known as canker sores, they are painful, recurring lesions of the oral mucous membranes. This is the most common lesion affecting oral mucosa; occurring up to 20% of the general population and 60% of select group (24). They are typically very tender and can become painful enough to interfere with speech, mastication, or swallowing.
Both oral and vulvar aphthae have been classified into three groups;
- Minor: they are smaller than 1 cm and heals within 7-10 days without scarring.
- Major: they are greater than 1 cm, deep, extremely painful, and heal with scarring in 10 to 30 days.
- Herpetiform: they are multiple, grouped, small ulcers that heal without scarring, and despite the name, are not associated with HSV.
Aphthae are classified further based on clinical course:
- Simple aphthosis: they have several episodes per year of either minor, major or herpetiform aphthae separated by disease-free periods.
- Complex aphthosis: they are defined as either almost constant presence of three or more ulcers, or recurrent oral and genital aphthae and exclusion of Behҫhet's disease (BD).
In the case of vulvar aphthae, keratinized, hair-bearing, as well as mucosal skin can be involved. Reported sites include the vagina, introitus, fourchette, labia minora, labia majora, and perineal body. The most common location is the medical aspect of the labia minora, often presenting bilaterally on opposing labial surfaces as "kissing aphthae" are typically 1 to 2 mm deep with well-demarcated, ragged borders. Borders can become overhanging and heaped up, sometimes simulating an ulcerated malignant tumor. The base of the ulcer ranges from black, necrotic tissue, to gray or yellow adherent exudates. The surrounding skin is usually erythematous, edematous, warm, and tender. Associated regional lymphadenopathy and cellulitis can occur.
They can occur in adult women but are more common in adolescent or premenarchal girls who are neither sexually active/abused, nor immunocompromised. Their discovery can be alarming and often precipitates an anxiety-provoking, costly, and unsuccessful search for sexual abuse or sexually transmitted infections. Patients and even many healthcare providers do not recognize non-infectious causes of vulvar ulcer; education about this entity is essential.
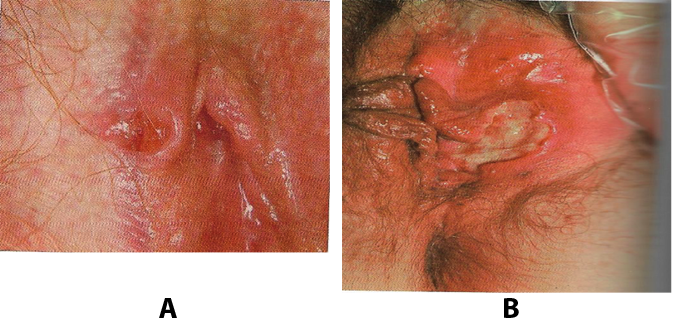
Figure 12. A:Vulvar aphthous ulcer in a teenage girl. B: Vulvar aphthous ulcer in a woman.
Pathogenesis
Lipschutz first reported vulvar aphthae in 1913 in adolescent girls in whom no specific cause was identified. Many authors have questioned the association with a viral infection, based on frequently associated antecedent fever, myalgias, and malaise. Epstein-Barr virus (EBV) has been most commonly implicated (25). Various theories have been postulated to explain the development of vulvar ulcers associated with EBV. EBV is a ubiquitous virus, and both the oropharynx and genital tract have been shown to harbor EBV even in the absence of symptoms. Therefore, direct transmission from sexual partners, either by genital - genital or oro-genital contact, has been suggested as the cause.
Other viruses have been implicated in vulvar aphthae are cytomegalovirus (CMV) and influenza A. Non-infectious causes also have been sought to explain vulvar aphthae in young girls. Friction and occlusion from tight-fitting clothing has been proposed as a physical cause in this group.
Evaluation of Vulvar Aphthae
The most important aspect of the evaluation a patient with vulvar aphthae is a complete history and physical examination, with particular attention to signs or symptoms of an underlying associated systemic condition: Behҫhet's disease (BD), HIV, malabsorption, ulcerative colitis, Crohn's disease (CD), celiac disease, cyclic neutropenia, periodic fever syndromes, and leukemia. An extensive laboratory work-up is often undertaken, creating great anxiety on the part of the patient and parents, and generating enormous unnecessary medical expense. Excluding HSV with viral culture or PCR is prudent. In sexually active or high-risk patients, screening for syphilis and HIV is appropriate. LGV, granuloma inguinale and chancroid are rare in the United States and should only be pursued in cases with a high index of suspicion. If biopsy is performed a full-thickness punch or incisional specimen that includes skin from the periphery of the ulcer, rather than from the center of the lesion. In a high-risk patient in whom infection is suspected, biopsy with special staining for organisms can be helpful way to make the diagnosis.
Laboratory evaluation of chronic vulvar aphthae should include HSV PCR or culture and HIV testing. Neutropenia and hematologic deficiencies should be ruled out with a complete blood count with differential, serum iron, folate, zinc, and vitamin B12. Consider searching for celiac with antibody testing and HLA-B27 for BD (26).
Treatment of Vulvar Aphthae
Many treatments for oral aphthae have not been rigorously studied and for vulvar aphthae have not been studied at all. Therefore, treatment of vulvar aphthae is based on that of oral aphthae, case reports, and mostly anecdotal experience. National registries and randomized trials are needed to assess the benefit of various treatment modalities.
Recurrent aphthous ulcers are the most common cause of recurrent oral ulceration in otherwise healthy individuals. Most people with recurrent aphthous ulcers develop a few ulcers less than 10 mm in diameter that heal after 7 to 10 days without scarring.
Key points in management are (27):
- In 10% of sufferers, lesions are more than 10 mm in diameter and can cause scarring.
- The majority of aphthous ulcers are idiopathic, although factors such as local physical trauma may trigger ulcers in susceptible people.
Chlorhexidine mouth rinses may reduce the severity and pain of ulceration, although studies have reported inconclusive results about whether the incidence of new ulcers is reduced.
Topical corticosteroids may reduce the number of new ulcers, reduce pain, and increase healing of ulcers without causing notable adverse effects.
About the effectiveness of local analgesics or tetracycline mouthwash, the evidence is weak. Use of topical antibiotics has not been studied; however, may case reports describe using oral antibiotics for presumed infection during management of acute vulvar aphthae.
II. Bechet's Disease (BD)
Behҫhet's disease (BD) is a chronic inflammatory vasculitis that can affect multiple systems. It was first described in 1937 by Hulusi Behҫhet from Istanbul. Mucocutaneous involvement is common, as is the involvement of many other systems such as the central nervous system and skin. BD can cause significant morbidity, such as loss of sight, and can be life threatening. The frequency of oral ulceration in BD is thought to be 97% to 100% (28). The presence of mouth ulcers can cause difficulties in eating, drinking and speaking leading to a reduction in quality of life. There is no cure for BD and therefore treatment of the oral ulcers that are associated with BD is palliative.
Pathogenesis
BD is characterized by a relapsing and remitting course. It manifests with oral and genital ulcerations, skin lesion, uveitis and vascular, central nervous system and gastrointestinal involvement. The main histopathologic finding is a widespread vasculitis of the arteries and veins of any size. The cause of disease is presumed to be multifactorial involving infectious triggers, genetic predisposition, and dysregulation of the immune system. The disease is present worldwide, but its prevalence is the highest in the Middle East, Mediterranean region and Asia. As in most complex diseases, both genetic and environmental factors are implicated in the pathogenesis of BD. HLA-B51 is by far the strongest associated genetic factor in patients with BD compared with controls ranging from 3.49 to 5.78 (29).
Recent data, however, from Italian and Turkish BD cohorts suggest that this association may be explained by a variant located between the HLA-B and MICA genes (OR=3.88) and not by the HLA-B51 (30). In addition to HLA-B51, several susceptibility genes within and outside the major histocompatibility complex have been described and are thought to play a role in this complex disease. These include HLA-A26, PSORS1C1, Cw1602, GIMAP, UBAC2, IL10, and IL12RB2, CCR1/CCR3, MEFV and TLR4.
Clinical Presentation
The usual age of onset is around 30 and the male to female ratio varies with the ethnic background, range of male/female patients 1:1 – 3:1. The disease burden of BD is confined to early years (around 15 years) of its course, and in many patient the syndrome burns out over the years. Major vessel disease and CNS involvement account for most of the deaths seen in this condition and can rarely appear for the first time relatively late in the course of the disease.
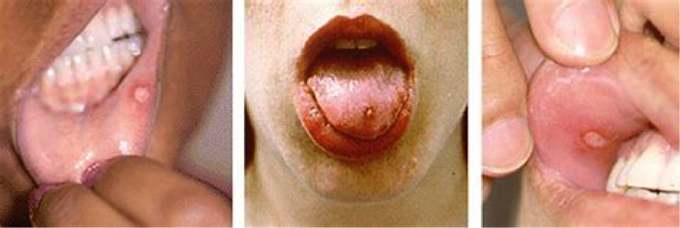
Figure 13. Behҫhet's oral disease; oral ulcerations.
Ocular manifestation
More than 50% of patients with BD have eye involvement, although it is much more common in males and younger patients. Eye involvement is usually not presenting feature of BD, but usually occurs withing initial few years of diagnosis, and is rare to occur in the disease if not present earlier.
Uveitis that is relapsing, chronic, bilateral, and involves both anterior and posterior uveal tracts is common. Anterior uveitis causes erythema and photophobia, while posterior uveitis causes vision loss. Hypopyon-related is less common but they very severe as it almost always accompanies the severe retinal disease. Retinal involvement with retinal vasculitis can be seen and is a cause of blindness in these patient. Conjunctivitis and isolated anterior uveitis are rare.
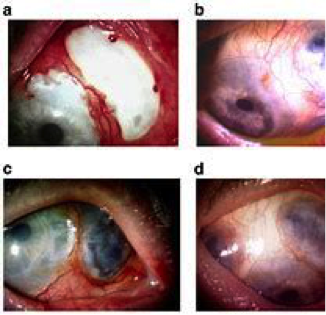
Figure 14. Behҫhet's ocular disease.
Cutaneous Manifestation
International study group criteria for BD were established in 1990 (31). By this consensus, patients must have recurrent oral aphthae at least 3 times in a year, plus 2 of the following:
- Recurrent genital aphthae;
- Uveitis or retinal vasculitis;
- Erythema nodosum-like lesions or populo-pustular skin lesions;
- Positive pathology test.
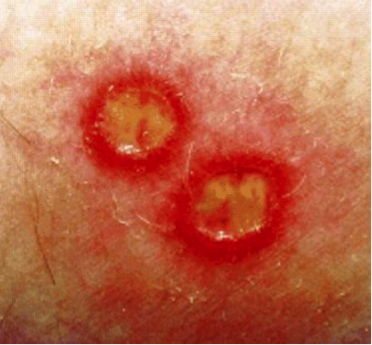
Figure 15. Typical cutaneous ulcers of Behҫhet's disease (BD); Genital ulcers are deep and painful, and eventually heal with scarring. Most common site is labia majora.
Ulcers involving oral and genital skin and mucosa are a hallmark of the disease. Second to oral ulcers, genital ulcers are the next most common features of BD, occurring in 57% to 93% of patients (31). Recognizing these lesions is essential because they may precede other features of the disease. Overlooking this association may result in delay in diagnosis and in increase in mortality. Aphthae associated with BD typically occur more frequently and more often in crops compared with recurrent aphthous stomatitis. Genital ulcers of BD typically start as a tender nodule, become deep and painful, and eventually heal with scarring. Searching for scars on genital skin, even in the absence of active, clinical disease, is an important part of the examination. Ulcers typically are found on the labia majora, but can occur anywhere on the vulva, perineum, or perianal skin. They can also be intravaginally, potentially leading to fistula formation with the urethra or bladder. Exceptionally deep external genital ulcers can lead to labial destruction.
Histopathology
Histopathological features of the disease are vasculitis and thrombosis. Biopsy of the mucocutaneous lesions shows a neutrophil-predominant reaction with endothelial swelling, extravasation of red blood cells, and leukocytoclastic vasculitis with fibrinoid necrosis of the blood vessel walls. The presence of lymphocytic vasculitis represents older lesions, and a neutrophilic vascular reaction is considered to be the most predominant reaction in Behҫhet's disease.
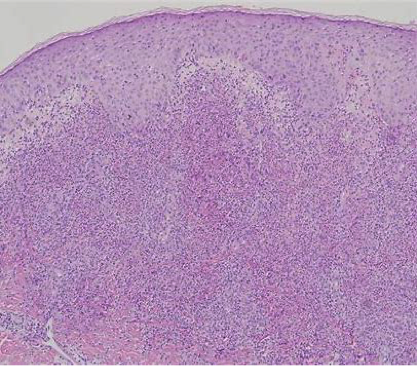
Figure 16. Biopsy of the mucocutaneous lesion shows a neutrophil-predominant reaction with endothelial swelling, extravasation of red blood cells, and leukocytoclastic vasculitis with fibrinoid necrosis of the blood vessel walls.
The involvement of vasa vasorum (vasculitis) may result in the formation of aneurysms in the large arteries. Synovial fluid analysis in Behҫhet's disease reveals neutrophil predominant leucocyte counts varying from 300 cells/mm3 (32).
Diagnostic Tests
The International Team for the Revision of International Criteria for Behҫhet's Disease (ITR-ICBD) revised the established criteria in 2008. The revised criteria are point-based and give 1 point each to oral aphthosis, skin manifestations (pseudofolliculitis, skin aphthosis), vascular lesions (phlebitis, large vein thrombosis, aneurysm, arterial thrombosis), positive pathology test and 2 point each to genital aphthosis and ocular lesions. 3 or more points are needed for the diagnosis of Behҫhet's disease (32). Similar to previous classification criteria, there are some pitfalls with this criteria as well.
Diagnosis of Behҫhet's disease is clinical and can be difficult due to lack of any pathognomic laboratory finding. Laboratory findings are usually non-specific, including anemia of chronic disease, leukocytosis, and elevation in markers of inflammation. Imaging studies shall be directed at the organ involved and may include x-rays and arthrocentesis to assess arthritis, CT-scan to assess for bleeding, thrombosis, and ischemia, angiography to look for aneurysms and lumbar puncture to evaluate meningitis. The rationale for carrying out these investigations is to rule out other causes of the clinical presentation. A careful ophthalmic examination to evaluate ocular involvement shall be pursued at initial presentation, and cutaneous lesions shall be biopsied to confirm cutaneous diagnosis.
Patients with inflammatory bowel disease, systemic lupus erythematosus (SLE), reactive arthritis, and herpetic infections can mimic Behҫhet's disease and shall be ruled out first.
Therapeutic options in Behҫhet's disease (BD)
The treatment is aimed at preventing organ damage. The treatment strategy is decided on organ involvement, severity, and prognostic factor. See table 4 below
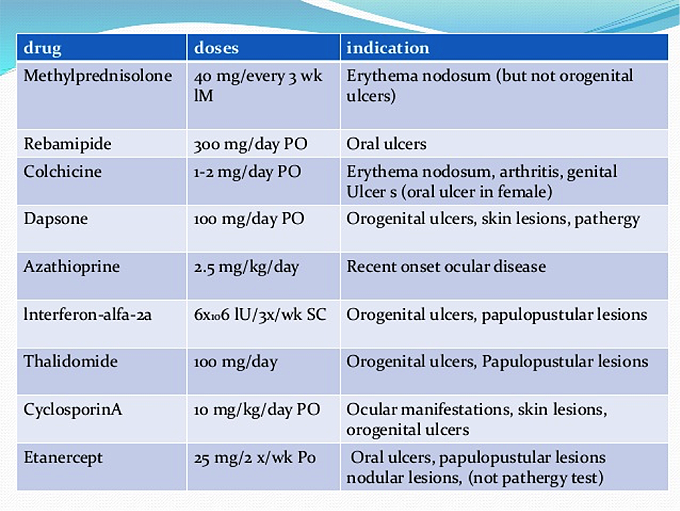
Table 4. Indications for the use of common therapeutic agents in Behҫhet's disease (BD).
Abbreviations: PO (orally); SC (subcutaneously).
Surgical treatment: Surgical interventions may be indicated in extensive vascular involvement refractory to medical management. Aneurysms tend to recur, and surgical intervention for aneurysms shall always accompany medical intervention to prevent recurrences. Surgery is also used to manage fistulas, intestinal stenosis, perforation, pulmonary artery aneurysms, glaucoma, cataracts and CNS aneurysms.
III. Crohn's Disease (CD)
Crohn's disease (CD) is a chronic relapsing and remitting inflammatory disorder of the gastrointestinal tract. The common presentation includes abdominal pain, abdominal cramping and diarrhea. Many patients may exhibit systemic symptoms of fever and weight loss. Approximately 20% to 40% of patients will experience extraintestinal manifestations that involve the eyes, skin and joints (33). Women may experience a variety of gynecological manifestations, including vulvovaginal involvement, which is often not recognized and also difficult to treat.
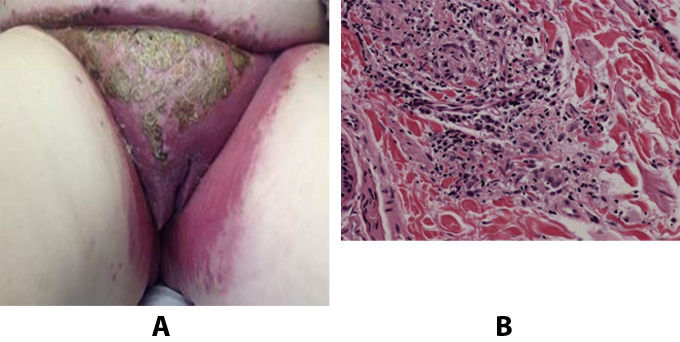
Figure 17. A: Extensive erythema, edema, and hyperkeratotic lesions of the vulva with Crohn's disease of vulva. B: Vulvar biopsy showing loose, ill defined, granulomatous inflammation in the dermis, consistent with cutaneous involvement by Crohn's disease.
Etiology
Cutaneous CD of the vulva is a rare condition with a diverse pattern of presentation. In patients without prior documented history of CD, the condition may not be considered for years. Moreover, due to the rarity of cutaneous CD, there are no well-established treatment strategies. Anogenital granulomatosis can have multiple etiologies. Underlying disease is detected in 38.4% of the patients, with the most common being CD 33% of the cases (34). CD is a systemic inflammatory condition, predominantly known for its effects on the gastrointestinal system. However, it has been estimated that cutaneous symptoms are present in 18 - 44% of patients with CD (34).
The development of vulvar lesions can occur as a direct extension of intestinal involvement or as a non-contiguous complication in which there is no direct connection between the gastrointestinal tract and genitalia. It has been reported that a T lymphocyte-mediated type IV reaction is responsible for the latter "metastatic" CD (35). Some suggest this reaction is to gut antigens that have traveled to the skin. Other hypothesize that antibodies sensitized to gut antigens may be antigens may be subsequently cross-reacting with skin antigens (35).
Clinical Presentation of Vulvar Crohn's Disease (CD)
The most common presentation of vulvar CD is edema, but hypertrophic lesions and also occur. Furthermore, one study found concomitant perianal lesions in 48% of the patients (36). Additionally, although most cases of vulvar CD cause minimal discomfort, this same study found vulvar pain in 34% of the patients, and pruritis in 9%, both significantly affecting the quality of life (36). In long-standing disease, patient may develop "knife-cut ulcers" that are highly specific for cutaneous CD (see figure 18 below).
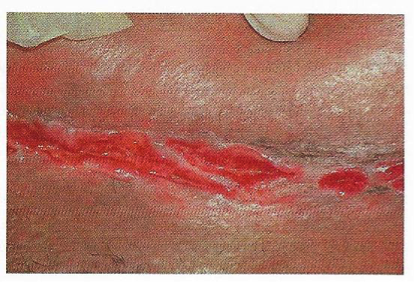
Figure 18. "Knife-cut" ulceration associated with Crohn's disease.
Further complicating the diagnosis, studies have that 20-36% of the patients with vulvar CD will not exhibit any gastrointestinal symptoms, and vulvar CD will be the first manifestation of their underlying disease. Not surprisingly, a preceding diagnosis of gastrointestinal disease increases the likelihood of a prompt diagnosis of cutaneous CD. Therefore a high index of suspicion is necessary for the timely diagnosis of vulvar disease, especially in patients with no prior documented history of CD.
Differential Diagnosis
Differential diagnosis of vulvar lesions are numerous, including infectious causes such as tuberculosis, LGV, syphilitic chancre, and herpetic lesions. It is also to consider non-infectious possibilities, such as pyoderma gangrenosum and sarcoidosis.
Serologic testing and immunochemical staining can rule out venereal disease. Tuberculosis screening with intradermal tuberculin reaction or the QuantiFERONTM-TB Gold test with a chest radiograph can evaluate for tuberculosis. Obtaining tissue biopsy of a lesion is a wise step after this initial workup, as special staining for microorganisms can further rule out infection. In addition, the presence of subacute or chronic inflammatory infiltrate with non-caseating granulomatous supports the diagnosis of cutaneous CD.
A chest radiograph can rule out the other common cause of non-caseating granulomas, sarcoidosis. If cutaneous CD is suspected, providers should arrange a gastroscopy and ileocolonoscopic with or without and abdominopelvic computed tomography (CT) scan to search for gastrointestinal CD. If no evidence of CD is found at that time, the disease can simply be labeled as anogenital granulomatosis. Treatment is similar regardless of diagnosis as cutaneous CD or anogenital granulomatosis of unknown origin.
Treatment
Studies have shown the natural course of vulvar CD is unpredictable. Though some cases resolve spontaneously, the clear majority are persistent. Further, earlier treatment is more effective, before the tissue has undergone reorganization secondary to long-standing edema. Because is rare, there are no randomized trials suggesting a particular treatment for vulvar CD. Further, the presently reported prospective studies and case series do not provide long-term follow-up data. Therefore, the literature presents no reliable recurrence rates following treatment.
Oral antibiotics, particularly metronidazole, have frequently been used as first line of therapy. Unfortunately, perineal CD does not respond well to antibiotics and are not always effective. Even if antibiotics are successful, rapid recurrence typically occurs when they are stopped (37).
Oral steroids are also frequently utilized in vulvar CD. Unfortunately, perineal CD has been shown to be poorly controlled with steroid. Many patients experience initial involvement, but their symptoms eventually return.
Immunosuppressants such as cyclosporine, azathioprine, and 6-mercaptopurine are also treatment options, as they have shown some effectiveness (37). In the event of moderate-to-severe refractory CD, TNF-α inhibitors are the new treatment of choice. Infliximab has been effective in cases that did not respond to other immunosuppressants including oral prednisolone, azathioprine, and cyclosporine (37).
Other biologics, adalimumab and certolizumab, have also shown effectiveness in resolving cutaneous CD. Multilayer compression therapy is also an option, as it has been shown effectiveness in decreasing vulvar edema of various causes.
Surgical intervention may be required for vulvar CD refractory to all medical therapies, such as fixed edema. Of note, localized surgical excision frequently results in localized recurrence and suboptimal wound healing, so radical vulvectomy is typically required in these difficult cases.
Summary
Causes of vulvar ulcers are diverse and can be divided into infectious and non-infectious categories. Most common causes of infectious vulvar ulcers are herpes simplex (HSV), syphilis, chancroid, LGV, granuloma inguinale, and HIV-associated vulvar ulcers in the United States. HSV is the most common of these. Non-infectious ulcers include those caused by drug reactions or adverse effects, autoimmune or inflammatory disease, trauma, and aphthous ulcers. Most common etiologies of non-infectious ulcers in the United States are: aphthous ulcers, Behҫhet's disease (BD) and Crohn's disease. Ulcers should be differentiated from erosions both clinically and histologically. This review described current diagnostic tests and management of these vulvar ulcers.
Suggested Reading
1. Sexually Transmitted Diseases
http://www.womenshealthsection.com/content/gyn/gyn013.php3
2. Genital Herpes Simplex Virus Infection during pregnancy
http://www.womenshealthsection.com/content/obsidp/obsidp003.php
3. Syphilis in Pregnancy: Prevention of Congenital Syphilis
http://www.womenshealthsection.com/content/obsidp/obsidp005.php3
4. Preventing Mother-to-Child Human Immunodeficiency Virus (HIV) Transmission
http://www.womenshealthsection.com/content/obsidp/obsidp010.php3
References
- Scheper T, Sascherbrecher S, Steinhagen K, et al. The glycoprotein C and G are equivalent target antigens for the determination of herpes simples virus type 1-specific antibodies. J Virol Methods 2010;166(1-2):42-47
- Freeman EE, Weiss HA, Glynn JR, Cross PL, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 2006;20(1):73-83
- Engel R, Carrell D, Krantz E, et al. Natural history of genital herpes simplex virus type 1 infection. Sex Transm Dis 2003;30(2):174-177
- Hofstetter AM, Rosenthal SL, Stanberry LR. Current thinking on genital herpes. Curr Opin Infect Dis 2014;27(1):75-83
- Anzivino E, Fioriti D, Mischitelli M, et al. Herpes simplex virus infection in pregnancy and in neonate: status of art of epidemiology, diagnosis, therapy and prevention. Virol J 2009;6:40
- Saurbrei A. Herpes genitalis: diagnosis, treatment and prevention. Geburtshilfe Frauenheilkd 2016 Dec; 76(12): 1310–1317. Published online 2016 Oct 18. doi: 10.1055/s-0042-116494
- De Clercq E. Selective anti-herpesvirus agents. Antivir Chem Chemother 2013;23(3):93-101
- Le Cleach L, Trinquart L, Do G, et al. Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients (review). Cochrane Database Syst Rev 2014;(8):CD009036
- Lafond RE, Lukehart SA. Biological basis for syphilis. Clin Microbiol Rev 2006;19(1):29-49
- Quatersooz P, Pierard GE. Skin homing of Treponema pallidum in early syphilis: an immunohistochemical study. Appl Immunohistochem Mol Morphol 2009;17(1):47-50
- Radolf JD, Tramont EC, Salazar JC. In: Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. Bennett JE, Dolin R, Blaser MJ, editors. 2014. pp. 2984-2709
- Castro R, Aguas MJ, Batista T, et al. Detection of Treponema pallidum Sp. Pallidum DNA in cerebrospinal fluid (CSF) by two PCR technologies. J Clin Lab Anal 2016;30(5):628-632
- World Health Organization. The Global Health Observatory. Data on syphilis. Geneva, Switzerland. Available @ https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/data-on-syphilis Last retrieved 18 September 2020
- Newman L, Rowley J, Vander Hoorn S, et al. Global estimates of the prevalence and incidence of Four Curable Sexually Transmitted Infections in 2012 based on systematic review and global reporting. PLoS One 2015;10(12):e0143304
- World Health Organization. WHO guidelines for the treatment of Treponema pallidum (syphilis). Geneva, Switzerland. 2016; available @ https://www.who.int/reproductivehealth/publications/rtis/syphilis-treatment-guidelines/en/ Last retrieved 20 September 2020
- Lewis D. Chancroid: clinical manifestations, diagnosis, and management. Sex Transm Infect 2003;79(1):68-71
- Roest RW, van der Meijden WI, European Branch of the International Union against Sexually Infection and the European Office of the World Health Organization. European guidelines for the management of tropical genito-ulcerative diseases. Int J STD AIDS 2001;12 Suppl 3:78-83
- White J, O'Farrell N, Daniels D, British Association for Sexual Health and HIV. 2013 UK National Guidelines for the management of lymphogranuloma venereum: Clinical Effectiveness Group of the British Association for Sexual Health and HIV (CEG/BASHH) Guideline development group. Int J STD AIDS 2013;24(8):593-601
- Morton AN, Fairley CK, Zaia AM, Chen MY. Anorectal lymphogranuloma venereum in a Melbourne man. Sex Health 2006;3(3):189-190
- Ceovic R, Gulin SJ. Lymphogranuloma venereum: diagnostic and treatment challenges. Infect Drug Resist 2015;8:39-47
- O'Farrell N. Donovanosis. Sex Transm Infect 2002;78:452-457
- Serwadda D, Gray RH, Sewankambo NK, et al. Human immunodeficiency virus acquisition associated with genital ulcer disease and herpes simples virus type 2 infection: a nested case-control study in Rakai, Uganda. The Journal of Infectious Diseases 2003;188:1492-1497
- Bandow GD. Diagnosis and management of vulvar ulcers. In: Vulvovaginal Dermatology; 2010; volume 28: Number 4, pp. 759
- Jurge S, Kuffer R, Scully C., et al. Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral Dis 2006;12(1):1-21
- Halvorsen JA, Brevig T, Aas T., et al. Genital ulcers as initial manifestation of Epstein-Barr virus infection, two new cases and review of the literature. Acta Derm Venereal 2006;86(5):439-442
- Pastore L, Carroccia A, Compilato D., et al. Oral manifestations of celiac disease. J Clin Gastroenterol 2008;42(3):224-232
- Staines K, Greenwood M. Aphthous ulcers (recurrent). BMJ Clin Evid 2015;2015:1303 PMC4356175
- Taylor J, Glenny AM, Walsh T., et al. Interventions for managing oral ulcers in Behҫhet's disease. Cochrane Database Syst Rev 2014;(9): CD011018; PMC6872426
- Saleh Z, Arayssi T. Update on the therapy of Behҫhet's disease. Ther Adv Chronic Dis 2014;5(3):112-134
- Hughes T, Coit P, Adler A., et al. Identification of multiple independent susceptibility loci in the HLA region in Behҫhet's disease. Nat Genet 2013;45(3):319-324
- Alpsoy E, Zouboulis CC. Ehrich GE. Mucocutaneous lesions of Behҫhet's disease. Yonsei Med J 2007;48(4):573-585
- Adil A, Goyal A, Bansal P, Quint JM. Behҫhet's disease. Stat Pearls Last update July 4, 2020. NIH.gov/books/NBK470257
- Makhiha S, Trotter M, Wagner E, et al. Refractory Crohn's disease of vulva treated with infliximab: A case report. Can j Gastroenterol 2007;21(12):835-837
- Van de Sheur MR, van der RI, van der Waal I, et al. Ano-genital granulomatosis: the counterpart of oro-facial granulomatosis. J Eur Acad Dermatol Venereol 2003;17(2):184-189
- Bender-Heine A, Granthtam JT, Zaslau S, Jansen R. Cutis 2017;99(6):e33-e40
- Barret M, de Parades V, Battistella M, et al. J Crohn's Colitis 2014;8(7):563-570
- Wells LE, Cohen D. Delayed diagnosis of vulvar Crohn's disease in a patient with no gastrointestinal symptoms. 2018;10(3):263-267
Published: 6 October 2020
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


