Intrauterine Contraception
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).
Intrauterine device (IUD) is the second most popular contraceptive method worldwide, after sterilization. Today's women have more birth control options than ever before. And with the increased options come increased expectations. Women want contraception that is safe, effective, convenient, and has few unwanted side effects. Intrauterine devices (IUDs) to prevent pregnancy have been in use for centuries. Although intrauterine contraception (IUC) is the most widely used reversible method of fertility regulation in the world today, only 1.3% of U.S. women aged 15 to 44 were using IUC in 2002 (1). However, since the US introduction of the levonorgestrel-releasing intrauterine system (LNG-IUS) in 2001, use of these contraceptives has increased substantially. Because of their convenience and effectiveness, interest in IUC is now growing rapidly. The LNG-IUS and copper-bearing IUC device rival sterilization in terms of efficacy, giving women an attractive alternative to permanent surgical sterilization. Indeed, in several countries the increased use of the LNG-IUS corresponds to a decrease in tubal sterilization and hysterectomy. Intrauterine contraceptives are safe and have been in use for many years. Both (copper and hormonal) IUC methods approved by Food and Drug Administration (FDA) feature a low risk of ectopic pregnancy, pelvic infection, and uterine perforation. Adherence and continuation largely determine the effectiveness of any contraceptive method. Intrauterine contraception frees women from adherence concerns, and its continuation rates exceed those for other types of hormonal contraception (eg, oral contraceptives). This should lead to greater utilization and fewer unplanned pregnancies.
The purpose of this document is to discuss evidence regarding the safety and efficacy of the levonorgestrel intrauterine system (LNG-IUS) and copper-bearing (TCu380A) intrauterine contraception. To achieve more widespread use of IUDs among women who are appropriate candidates, health care providers should understand the risks, benefits, indications, and contraindications of IUD use. Two IUDs currently are available in the United States: 1) the copper TCu380A, and 2) the levonorgestrel intrauterine system (LNG-IUS). A growing body of evidence attests to the safety and effectiveness of IUDs and their potential role in rates of unintended pregnancy.
Historical Perspective:
IUD technology has come a long way since the first plastic IUDs (the Lippes Loop, Margulies Spiral, Saf-T-Coil, and others) appeared on the scene in the
1960s. Towards the end of that decade, researchers discovered that adding copper to the plastic produced an IUD that was more effective in preventing pregnancy and caused bleeding problems less frequently. The first copper-bearing IUDs—Copper-7, TCu-200, and Nova T—appeared in the early 1970s, but they required replacement every two or three years. Further research at the end of the 1970s produced second-generation copper IUDs carrying larger quantities of copper: among the better known examples are TCu380A, TCu-220C, and Multiload-375. These devices not only reduced the incidence of side effects compared with previous IUDs but also had significantly lower failure rates. Meanwhile, in the mid-1970s, Program of Development and Research Training in Human Reproduction (HRP) at World Health Organization (WHO) had entered the scene in response to the need for independent well-designed studies that would enable the scientific and public health communities to judge the relative merits of the plethora of devices available at the time and would help governments and non-governmental organizations (NGOs) to make informed choices about which devices to include in their family planning programs (2).
In the mid-1980s, however, the popularity of IUDs plummeted when researchers linked one IUD, the Dalkon Shield, to relatively frequent septic abortions (i.e. abortions or threatened abortions associated with pelvic infection) in the second trimester of pregnancy. This IUD, which was launched in 1971 in the USA, was withdrawn in 1974 in the face of litigation and adverse press coverage. The whole IUD market, however, became tarnished with the Dalkon Shield brush -- its use was linked to pelvic inflammatory disease (PID), septic spontaneous abortion, infertility, and even death. WHO convened a scientific group of experts in 1986 to address this issue -- they concluded that "the use of IUDs in both developed and developing countries should continue to be supported as a reliable and safe method of reversible fertility regulation" (3). The experts also observed that the newer copper devices, notably Multiload-375 and TCu380A, were, after two years' use, significantly better at preventing pregnancy than their predecessors. They also judged that results that had become available from long-term HRP studies justified an extension of the life span of these copper IUDs from two to at least five years. By the end of 2001, three IUDs had emerged from the fray—TCu380A, Multiload-375, and Mirena® (or LevoNova) -- a levonorgestrel-releasing device introduced in 1984—and HRP was still fielding three long-term international multicenter trials involving large cohorts of women. Worldwide, IUDs are the most common reversible method of contraception and are used by more than 90 million women. The largest numbers of women, who use IUD as the contraception, reside in China -- where 40% of women who use the contraception use the IUD. Other countries with high rates of IUD use include Vietnam, Norway, Finland and Sweden (3).
Overview of Intrauterine Contraceptives (IUCs) Currently Available in USA:
Most modern are IUDs medicated, containing either copper or a progestin to enhance the contraceptive action of the device. These medicated IUDs are referred to as intrauterine contraceptives (IUC). The progestin-containing device is also referred to as an intrauterine system (IUS). There are three categories of modern IUCs:
- Copper IUCs;
- Progestin-releasing IUCs;
- Unmedicated (inert) IUD
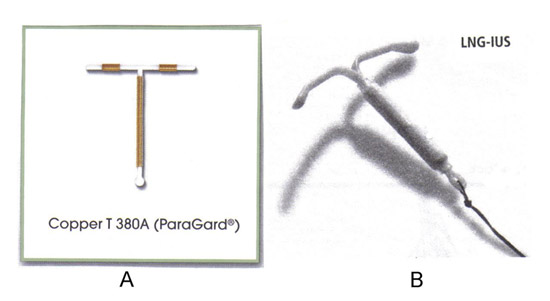
The two types of IUCs currently available in the United States are, the TCu380A (ParaGard®) and the levonorgestrel releasing IUC (Mirena® LNG-IUS). However, additional copper and inert IUDs are available worldwide. One example is the frameless IUC (GyneFix®), which is made of copper tubing attached to a nylon thread (4). A frameless IUC is available in several countries, but not the United States. It is made up of several copper cylinders without the plastic frame common to other IUCs. A frameless LNG-IUS is also in the development. Early generation frameless devices were associated with a high rate of expulsion, but subsequent introducer systems that anchor the device to the myometrium appear to have overcome this problem. These frameless IUCs are as effective as conventional IUCs and probably more adoptable to variations in the shape of the uterine cavity (4).
nThe chart below provides an overview of the two IUCs that are currently available in the US (5):
| Parameters | Copper TCu380A (ParaGard®) | LNG-IUS (Mirena®) |
|---|---|---|
| Effectiveness Duration of therapeutic effect, years First year of use pregnancy rate, perfect use First year of use pregnancy rate, typical use 7 year cumulative pregnancy rate, 10 year cumulative pregnancy rate, |
>99% 12 0.6% 0.5 to 0.8% 1.6% 2.2% |
>99% 7 0.1% 0.1% to 0.2% 1.1% ----- |
| Hormonal | No; Releases copper |
Yes, Releases levonorgestrel |
| Length of use | Up to 10 years of reversible contraception | Up to 5 years of reversible contraception |
| Use in nulliparous women | Yes, May be used in women regardless of parity |
No, Recommended for women who have had at least one child |
| Use in women with a history of PID | Yes, However, it should not be used in women with acute PID or current behavior suggesting high risk for PID |
No, Acute PID or history of PID are contraindications unless there has been a subsequent intrauterine pregnancy. |
Mechanism of Action:
A number of different mechanisms of action have been proposed for copper-containing IUDs. These include inhibition of sperm migration and viability, change in transport speed of the ovum, and damage to or destruction of the ovum. The evidence suggests these pre-fertilization effects constitute the primary mechanism of action for pregnancy prevention in the copper IUD. Post-fertilization effects, including damage to or destruction of the fertilized ovum, also may occur (6). In addition to these effects, the LNG-IUS causes endometrial suppression and changes the amount and viscosity of cervical mucus. All effects, both pre-fertilization and post-fertilization, occur before implantation. To summarize the current scientific mechanism of action of IUCs:
Effect on sperm:
- Sterile foreign body reaction in uterine cavity results in biochemical and cellular changes that may be toxic to sperm;
- Copper released from TCu380A is spermicidal or cytotoxic;
- Thickening of cervical mucus by LNG-IUS may impede sperm transport through the cervix (6).
Effect on fertilization:
- Marked decrease in amount of fertilized ova found in fallopian tubes of IUD users compared to women not using contraception.
Effect on Endometrium (6):
- TCu380A increases leukocytes in the endometrium;
- Both IUCs alter cytokines and integrins in the endometrial lining;
- LNG-IUS causes endometrial suppression, decreased thickness and secretions.
Counseling and Patient Selection:
Counseling should cover the risks and benefits of intrauterine contraception, associated side effects and their management, and availability of alternative methods of contraception and their risk and benefits. Clinical experience has shown that much of the patient dissatisfaction that results from method-related side effects can be averted by a thorough discussion of contraceptive methods prior to initiation. In particular, training in counseling about intrauterine contraception may enhance patient satisfaction (7). The intrauterine contraception is an excellent choice for most women because it is safe, highly effective, private, coitus-independent, long-acting, rapidly reversible, cost-effective, and has few side-effects. Ideal candidates for IUC are women who: are at low risk of sexually transmitted diseases (STDs); are not planning a pregnancy for at least one year; want to use a reversible contraceptive; and want or need to avoid estrogen-based methods. Use of the IUC in other populations is also reasonable and may be indicated. As an example, the LNG-IUS is an excellent choice for women with heavy menstrual flow since it significantly reduces bleeding. Nulliparous women, women in non-monogamous relationships, adolescents, breastfeeding women, and women with history of PID or ectopic pregnancy can all safely use the IUC with appropriate counseling about side effects and prevention of sexually transmitted diseases.
Women with the following medical conditions, for which an intrauterine device may be an optimal method (8): diabetes; thromboembolism; menorrhagia; dysmenorrhea; breastfeeding; breast cancer; and liver disease. Women using IUD as their family planning method should understand when to return for follow-up evaluation and should be instructed in checking for the strings of the IUD. Generally, women should be re-evaluated 1-4 weeks after IUD placement. For women who use the copper TCu380A, a missed period should prompt a pregnancy test; a positive pregnancy test result should prompt an immediate visit to a provider to rule out ectopic pregnancy. Amenorrhea in women using the LNG-IUS is common. However, in a woman who misses a period and experiences pain, ectopic pregnancy should be ruled out. Women should be instructed about warning signs of pelvic infection, particularly in the first month after insertion of the device, when the risk of pelvic infection is increased.
Contraindications:
There are relatively few absolute contraindications to intrauterine contraception. When considering any method, its contraceptive and non-contraceptive benefits must be weighed against the risks, as well as the risks of unintended pregnancy with alternative methods of contraception.
- Severe uterine distortion -- anatomic abnormalities including bicornuate uterus, cervical stenosis, or leiomyomata distorting the uterine cavity are contraindications to use because of increased difficulty with insertion and increased risk of expulsion when an IUC is placed in a distorted, small or extremely large uterine cavity. Manufactures recommend that the uterine cavity be between 6 and 9 cm for optimal performance, but this is not based on good evidence. Non-distorting leiomyomata are not a contraindication to intrauterine contraception (8).
- Active pelvic infection -- IUC insertion in women with postpartum endometritis, post-abortion infection, or active STDs (including mucopurulent cervicitis) increases the risk of upper genital tract infection. There is no contraindication to use the IUC in a woman with a distant history of PID.
- Known or suspected pregnancy -- IUC insertion during pregnancy can lead to miscarriage and increases the risk of septic abortion.
- Wilson's disease or copper allergy -- the amount of copper released daily by the TCu380A is less than that consumed in an average daily diet. Although no adverse event related to copper allergy or Wilson's disease has ever been reported with a copper IUC, hormone-releasing IUCs are preferred for use in these patients (9).
- Undiagnosed abnormal uterine bleeding -- irregular bleeding may be erroneously attributed to the IUC. The evaluation and diagnosis of gynecologic problems (eg, cervical and endometrial cancer) should precede IUC placement. Cervical dysplasia or cervical ectropion are not contraindications to IUC.
- Current breast cancer -- it is contraindicated to use LNG-IUS in these patients.
- Relative contraindications -- IUC should be used with caution in women with increased risk for STDs (including having more than one partner or a partner with more than one partner) or a history of recently (within three months) treated gonorrhea or chlamydia. Women in non-monogamous relationships can decrease their risk of STDs by using condoms in addition to intrauterine and other contraceptives. Other relative contraindications include a current history of dysmenorrhea or menorrhagia (for the copper IUC only); previous problems with intrauterine contraception -- including pregnancy, expulsion, perforation, pain, or heavy bleeding, are not contraindications to use; however, the woman should be informed of other options and counseled. The LNG-IUS has additional relative contraindications related to specific hormonally sensitive conditions.
Intrauterine Contraceptives (IUCs) and Risk of Pelvic Inflammatory Disease (PID):
A disconnect exists between the perception of risk of PID with IUCs and data on the actual risk. An analysis of 12 randomized WHO clinical trials on IUC indicates that the incidence of PID for IUC users is very low and is most strongly related to insertion and background rate of STDs, rather than the IUC itself (10). Among 22,908 IUC insertions evaluated in this analysis, the incidence of PID was 1.6 cases per 1,000 woman-years (10). The highest incidence of PID was shown to occur within 20 days of insertion (9.6 cases per 1,000 woman-years) with the rate dropping dramatically beyond this time (1.38 cases per 1,000 woman-years >21 days after insertion) and remaining low for up to 8 years of follow-up. The data suggest that those IUC users at low risk for STDs do not have an excess rate of PID. Nevertheless, clinicians need to keep in mind that pelvic infection is commonly associated with sexually transmitted organisms (eg, chlamydia and gonorrhea), and women at high risk for STDs are not appropriate candidate for IUCs.
Routine testing for STDs prior to IUC insertion and what to do if a current IUC user develops an STD are topics that merit discussion. A clinician's decision to test for STDs prior to IUC insertion should be based on a thorough history and examination of the woman. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on IUCs suggests that routine screening in women at low risk for STDs is not necessary (11). The appropriateness of IUD use in nulliparous women is controversial, largely because of fears of PID with subsequent infertility. Historically, the labeling for IUCs suggested that this method was recommended for women who had already had children, resulting in a barrier to use in nulliparous women. This recommendation was removed from the labeling for ParaGard®; however, the Mirena® labeling still contains a statement that it is recommended for women who have had at least one child. ACOG indicates that IUCs are appropriate for many nulliparous and adolescent women (11). Multiple studies have discounted this contention by reporting no difference in risk of tubal infertility or PID between nulliparous women and parous women. In a study conducted by Family Health International and the National Perinatology Institute to evaluate the association of tubal infertility with copper IUC use among nulligravid women, use of a copper IUC was not associated with an increased risk of tubal occlusion, compared with a control group of infertile women and pregnant women (12). Among these nulligravid women there was a significant increased risk of tubal occlusion in those with antibodies toChlamydia trachomatis, compared with pregnant controls, implicating STDs rather than IUC use as a risk factor for infertility.
Asymptomatic Patient with IUD and Actinomyces identified on a Pap Test:
Actinomyces israelli, a gram-positive anaerobic bacterium normally found in the human gastrointestinal tract, may be a normal component of vaginal flora. This organism may be more prevalent in the genital tract of IUD users than in non-users. The likelihood to colonization appears to increase with duration of IUD use. Recent studies demonstrated that colonization may be lower in LNG-IUS users than in copper IUD users (2.9% versus 5-10%) (13). However, actinomyces found via a Pap test is not diagnostic of actinomycosis infection, nor it is predictive of future disease. Pelvic actinomycosis is a very rare but serious condition characterized by granulomatous pelvic abscesses. Its prevalence has been estimated to be less than 0.001%; because of its rarity, the relationship between actinomyces found on a Pap test in an asymptomatic IUD user and the eventual development of this infection is unclear (11). Studies of pelvic actinomycosis are limited to case reports, so management of the asymptomatic IUD user whose Pap test shows actinomyces is not clearly established. A single randomized controlled trial has looked at management of asymptomatic IUD users with actinomyces identified in Pap test (14). Women were randomized to undergo either removal of the IUD and receive oral antibiotics or receive oral antibiotics alone. One month after treatment, the Pap test was repeated. No Pap tests revealed actinomyces in the women whose IUDs were removed. 33% of Pap test still showed actinomyces in the group of women who received antibiotics alone. However, the importance of clearing the actinomyces colonization is still not established. The options for management of asymptomatic IUD users with actinomyces on Pap test are expectant management, an extended course of oral antibiotics, removal of IUD, and both antibiotic use and IUD removal.
IUD Use and Ectopic Pregnancy:
Ectopic pregnancies are unlikely to occur in IUD users because pregnancy is so rare. Using an IUD does not increase a woman's risk for ectopic pregnancy, although a rare pregnancy that occurs with an IUD in place is more likely to be an ectopic pregnancy compared to the general population. Approximately 3 to 5% of all contraception failures with an IUD are ectopic; the rate is slightly higher in LNG-IUS users (15). TCu380A users are 90% less likely to have an ectopic pregnancy than women not using contraception. The 12-year cumulative ectopic rate for the TCu380A is 0.4%. If pregnancy occurs with an IUD in situ, the site of pregnancy should be determined immediately. Adopted from United States Food and Drug Administration reviews of data submitted by drug manufacturers to support marketing approval of the incidence of ectopic pregnancies as a proportion of all pregnancies by contraceptive method is listed below (15):
| Contraceptive Method | Ectopic Pregnancy/ All Pregnancies |
|---|---|
| Levonorgestrel intrauterine device | 1:2 |
| Copper intrauterine device | 1:16 |
| Progesterone intrauterine device | 1:4 |
| Tubal sterilization | 1:3 |
| Norgestrel-only pill | 1:21 |
| Norethindrone-only pill | 1:20 |
| Combination pills | 0 |
| All women | 1:50 |
Hormonal Side-effects:
Hormonal side effects (including hirsutism, acne, weight change, nausea, headache, mood changes, and breast tenderness) are the most common reasons for elective LNG-IUS removal in the first 36 months of use (16). LNG-IUS users also have more discontinuations due to hair and skin changes and headache than users of copper IUCs. These complaints may be due to systemic effects of levonorgestrel, even though plasma levonorgestrel levels are low (16). Premature LNG-IUS discontinuation for hormonal complaints occurs in approximately 12% of women over five years. Small functional ovarian cysts have been reported in 12% of LNG-IUS users; the cysts usually are asymptomatic and resolve over a few years.
Insertion and Removal:
Rather than limiting IUD insertion to the first 7 days after normal menses, a provider can insert an IUD at any time, given reasonable assurance that the woman is not pregnant. Documentation of a negative pregnancy test is recommended for women with unclear ovulation timing or questionable compliance with contraception. The pregnancy test should be obtained at least 2 weeks after the last episode of unprotected intercourse. The onset of contraceptive action varies by device. The copper TCu380A is effective immediately after insertion and can also be used as emergency contraception for women within 5 days of a single act of unprotected intercourse. The copper TCu380A is also more effective than emergency hormonal contraception (24). The LNG-IUS is effective immediately only if inserted within 7 days of the onset of menstruation. If the device is inserted more than 7 days after menses, back-up contraception with condoms or abstinence should be used for 7 days.
Placement of IUC can be performed in the health care provider's office during a routine visit. In general, no anesthesia or sedation is required, although a cervical block can be performed if needed. Use of the over the counter non-steroidal anti-inflammatory (NSAID) drugs in advance of the procedure may be beneficial to alleviate cramping on placement and subsequently. IUC insertion is safe immediately after spontaneous or induced abortion and is not associated with an increased risk of perforation or infection. There are higher rates of expulsion of the IUC if inserted immediately after a second trimester abortion. If a woman desires an IUC immediately after an abortion and is unlikely to return for insertion, post-abortion insertion is a reasonable option. Either the TCu380A or LNG-IUS may be used. However, an IUC should not be inserted if there is evidence of an active infection. The TCu380A is the only IUC currently recommended for postpartum insertion. A systematic review showed that compared to interval insertion, postpartum insertion appeared to have a higher rate of expulsion, but no increase in perforation or infection (17). Expulsion is less likely when insertion is performed within 10 minutes of delivery of the placenta versus one to two days postpartum. If immediate postpartum insertion is not done, then waiting four to six weeks is advisable.
Antibiotic prophylaxis before IUD insertion is unlikely to be cost-effective in populations with a low prevalence of STDs. Bacteremia is uncommon in clinically well women who undergo IUD insertion and removal. Therefore, on the basis of expert opinion, the American Heart Association does not recommend sub-acute bacterial endocarditis antibiotic prophylaxis for IUD insertion and removal (11). IUDs can be removed either at the end of its life-span or sooner if the patient wishes to discontinue contraception. For removal of IUD -- the healthcare provider applies gentle traction on the threads of IUD extending outside the cervix, the arms of the IUD folds upward and the device slides out of uterine cavity. This only takes few seconds and is well tolerated in office settings. Routine sonography is not indicated following placement or at other times in women using IUDs. However, vaginal sonography is invaluable for patients when the position of the IUD needs to be evaluated. For example, ultrasound can verify the device's correct fundal position if the threads are not visible, if the IUD has moved on the vaginal speculum examination or if expulsion (partial or complete) is suspected. A copper IUC inserted for contraception should be removed one year after the last menstrual period in menopausal women, but the LNG-IUS will provide protection from endometrial hyperplasia for women who elect to use estrogen therapy. This protection extends for at least 10 years after the date of insertion.
n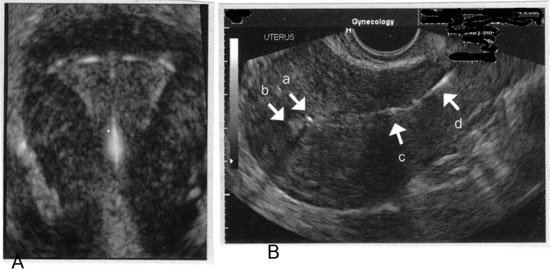
Imaging of intrauterine contraceptive devices with transvaginal ultrasound. A: 3-D image of appropriate fundal position of IUD; B: With this sagittal view, the superior component of the device (a) is imaged in proximity to the fundal portion of the endometrium, (b) confirming appropriate fundal position of the IUD. The inferior component of the device (c) is imaged in the lower uterine segment, and the threads (d) are imaged in the upper portion of the endocervical canal.
Use and Efficacy of IUC:
TCu380A (copper) IUC is approved to remain in place for10 years, but is effective for at least 12 years. With perfect use (in which the user checks the strings regularly to detect expulsion), the probability of pregnancy in the first year is 0.6%; with typical use, the first-year pregnancy rate is 0.5 to 0.8%. After prolonged continuous use, the cumulative pregnancy rate is 1.6% at seven years, and 2.2% at 8 to 12 years. Overall, the failure rate is substantially less than one per 100 women per year, except in women under age 25 who experience a slightly higher failure rate, most likely because they are more fertile than older women. The TCu380A appears to be more effective than other types of copper IUCs and is the only copper IUC available in the United States. The LNG-IUS is approved for up to5 years of use in the United States, but is effective for up to seven years. With perfect use, the probability of pregnancy in the first-year is 0.1%, with typical use; the first-year pregnancy rate is 0.1 to 0.2% (1)(2). With seven years of continuous use, the cumulative pregnancy rate is 1.1%. The LNG-IUS, the TCu380A, and the single-rod implant are the most effective forms of reversible contraception available (1)(2). By comparison, the cumulative risk of pregnancy 10 years after tubal ligation is 1.9%.
Expulsion of IUC:
In the first year of use, expulsion occurs in 3 to 10% of women with the TCu380A and 6% of those with LNG-IUS (18). Risk factors for expulsion include: nulliparity; menorrhagia; severe dysmenorrhea; prior expulsion (30% chance of repeat expulsion); age less than 20 years; and insertion immediately after second trimester abortion or postpartum. Symptoms of IUC being expelled include cramping, vaginal discharge, intermenstrual or post-coital bleeding or spotting, male or female dyspareunia, lengthened or absent strings, or a palpable IUC in the cervix or vagina. If an ongoing expulsion is suspected, the woman should receive immediate medical attention. If there is no pregnancy or infection, and expulsion is complete, the provider may insert a new IUC.
Uterine Perforation:
Uterine perforation occurs in 1 in 1,000 IUC insertions, almost always at the time of insertion (19). Risk factors include an inexperienced clinician and an immobile and retroverted uterus. Pain is the primary clinical manifestation of perforation. The provider may note a loss of resistance while inserting the IUC or that the uterus sounds to an expected depth. If perforation is noted acutely, the IUC should be removed, if possible, by gently pulling on the strings. However, if there is resistance to removal, an immediate laparoscopy should be performed to remove IUC under direct vision so as not to injure pelvic organs that may be caught by the device. Perforation also can be relatively asymptomatic and may not be noted at the time of insertion. It is important, therefore, to check the strings within few weeks of placement. Ultrasound or if not available, x-ray can be used to detect the location of a displaced IUC. Expulsion can only be diagnosed if an x-ray is obtained and does not show an IUC. Although serious complications following perforation are rare, it is recommended that any perforated IUC be removed unless the surgical risk is excessive. Operative laparoscopy is indicated if the IUC is perforating the myometrium or is in the abdominal cavity, and can be performed electively in asymptomatic patients. If laparoscopy is unsuccessful due to extensive adhesions, the surgery should be converted to laparotomy. If the IUC is embedded in the uterine cavity, operative hysteroscopy may be required for removal. Once perforation has been identified, the woman should be treated with antibiotics as for PID.
Menstrual Disorders and Dysmenorrhea:
Both the TCu380A and the LNG-IUS are associated with changes in bleeding patterns. Counseling about expected changes in bleeding patterns prior to insertion may enhance adherence to this method. Unexpected changes in bleeding patterns or changes that are not tolerable to the patient should be evaluated. If a copper IUC user presents with new onset or persistent intermenstrual bleeding, the provider should exclude pregnancy (intrauterine and ectopic), infection, and partial expulsion, as well as assess for anemia (if clinically indicated) and gynecologic disorders of the cervix or uterus. Approximately 15 to 20% of women discontinue the TCu380A IUC within 12 months of insertion because of abnormal bleeding (20). A copper IUC should be removed if the woman complaints of menorrhagia and experiences an associated clinically significant fall in hemoglobin. The LNG-IUS or other contraception can be considered for these patients. Mean per cycle blood loss for copper and LNG-IUS is 55 and 5 mL, respectively (20). Short-term hormonal manipulation with oral contraceptive pills is unlikely to be effective and is not advised. In women over age 40 or with risk factor for endometrial cancer who develop irregular bleeding patterns, the endometrium should be evaluated. We advise removing the IUC prior to performing an endometrial biopsy.
The LNG-IUS is associated with both a reduction in menstrual blood loss and with episodes of unscheduled bleeding, which may be limited to spotting. The incidence of unpredictable bleeding is greatest in the initial six months of use, although episodes may occur throughout usage of the LNG-IUS. The proportion of users with amenorrhea increases with duration of use (20). At six month of use, 44% of users have amenorrhea, 25% experience oligomenorrhea, and 25% experience unscheduled spotting; the remainder has either normal or heavy bleeding. At 24 months of use, 50% have amenorrhea, 25% have oligomenorrhea, and 11% have spotting; again the remainder report either normal or heavy bleeding (5)(16)(20). Amenorrhea in LNG-IUS users is due to endometrial decidualization and atropy; at one year the majority of women have ovulatory cycles.
Emergency Contraception:
Insertion of copper-bearing IUD after unprotected coitus confers powerful protection against pregnancy, whereas the LNG-IUS should not be used for this purpose (24). One randomized controlled trial compared insertion of a copper T200 (200 women) with expectant management after unprotected sex (100 women) (21). As expected, the risk of pregnancy after IUD insertion was 10% of that in the watchful-waiting group. Consensus of opinion is that the TCu380A IUC can be placed within 120 hours of unprotected intercourse as a form of emergency contraception, and has efficacy of 98.1% in parous women and 92.4% in nulliparous women.
The LNG-IUS Usage with Tamoxifen:
Tamoxifen is often prescribed for women who have estrogen-receptor-positive breast cancer. In the uterus and endometrium, tamoxifen acts as a partial estrogen agonist and is associated with an increased risk of endometrial polyps and endometrial cancer. Placement of the LNG-IUS in a woman who is taking tamoxifen for breast cancer appears to reduce her risk of developing endometrial polyps. In a recently published trial, 113 women who had breast cancer and were taking tamoxifen were randomized to either 1) placement of an LNG-IUS plus endometrial surveillance (by transvaginal ultrasonography) or 2) endometrial surveillance only (22). After an average, approximately 25 months of follow-up, new polyps arose in eight women who had not been assigned to have the LNG-IUS placed. New polyps arose in three women randomized to receive the LNG-IUS, but notably none of those three had the LNG-IUS in place at the time the polyp was diagnosed: two, because they had had the device removed; one, because the device had in fact never placed. This finding might be an important development in women's health. The LNG-IUS likely reduces the risk of endometrial cancer and is a therapeutic option for hyperplasia with and without atypia. It may also be a therapeutic option for women with grade 1, stage 1 endometrioid uterine adenocarcinoma who are poor operative candidates. Although not approved by the FDA for this purpose, use of the LNG-IUS reduces the risk of endometrial hyperplasia in women exposed to unopposed estrogen.
IUD in Immunocompromised Women:
Intrauterine devices (IUDs) are a viable treatment option for immunocompromised women who need contraception or menses suppression. The literature on IUD use in immunocompromised women is sparse, but most of the available evidence is in the human immunodeficiency virus (HIV)-infected population. Some of the theoretical concerns about IUD placement in HIV-infected women include an increased risk of PID because they are immunocompromised, and a theoretical increase in the risk of female-to-male transmission of HIV by way of increased viral shedding or menstrual blood loss. However, data do not suggest that immunocompromised HIV-infected women have a higher likelihood of febrile morbidity with IUD usage or higher female-to-male HIV-1 transmission rate. A well-designed prospective study in 98 women examining the prevalence of HIV-1 DNA cervical shedding showed no significant difference in viral shedding rates at baseline (50%) and at 4-month follow-up after copper IUD insertion (43%) (odds ratio 0.8; 95% confidence interval [CI] 0.5-12) (23). In addition to the contraceptive benefits of IUDs, the LNG-IUS has been shown to reduce menstrual blood loss, with an associated slight increase in hemoglobin, during a 12-month follow-up in a small prospective study of 12 HIV-infected-women (23). No recurrence of pelvic inflammatory disease was reported in this study. Moreover, detectable rates of HIV RNA in cervicovaginal lavage remained the same before and after the insertion of the LNG-IUS (10%).
Copper IUD use in women with systemic lupus erythematosus (SLE) has not been associated with an increase in pelvic infections (9). Julkunen et al investigated the contraceptive practices of 85 women with SLE compared with immunocompetent women and found that there was a lower tendency for IUD and oral contraceptive use in women with SLE compared with barrier and natural methods. Although only 12% of women used a copper IUD in this study, there were no major infectious or bleeding complications associated with its use (23). The authors postulated that the lower use of IUDs by SLE patients as compared with healthy women may reflect the fear of both physicians and patients about the potential risk of developing infections because they are immunocompromised. LNG-IUS use for treatment of menorrhagia secondary to uterine myomas in a renal transplant patient has also been reported and was not associated with febrile complications (9)(23). More recent studies do not support an increased risk of infectious morbidity with IUD use in immunocompromised women, but its use should be closely monitored for abnormal clinical signs and symptoms in these women. Based on current available data, the IUD is a viable treatment option for immunocompromised women who need contraception and menses suppression, and it may be an alternative treatment for women who have a contraindication to estrogen use.
Pregnancy Outcome with Intrauterine Contraceptive Device:
Pregnancy in the presence of an IUD involves several complications; among the most noted is ectopic pregnancy. Further complications of pregnancy in the presence of an IUD include premature labor and abortion. Among women who conceive with an IUD that remains in situ, the risk of spontaneous abortion is 40-50%, a rate twice as high as that of the general obstetric population. The World Health Organization recommends that in the event of a pregnancy in the presence of IUD, the IUD be removed if the string is visible and the device can be removed through the cervix easily (2)(3). Recently a retrospective study comparing the pregnancy outcome of women with retained intrauterine device (n=98), patients after intrauterine device removal in early pregnancy (n=194), and pregnancies without an intrauterine device (n=141,191) was performed (25). The study concluded: women who conceive with an IUD in place are at increased risk for adverse obstetric outcomes. The risk is higher for pregnancies with a retained IUD than for those with early IUD removal. Women conceiving with an IUD in place should be informed regarding adverse maternal and perinatal outcomes demonstrated, seeing as IUD removal diminishes the risk for adverse obstetric outcomes, but does not abolish it. Careful surveillance of high-risk pregnancies and during the neonatal period is warranted. Further investigation is needed regarding the influence of gestational age at IUD removal and the length of IUD use before conception on the adverse outcomes demonstrated so that accurate recommendations can be made for women who conceive while using an IUD. Our recommendations are:
- Rule out ectopic pregnancy;
- If the pregnancy is intrauterine, within the first trimester, and the IUD strings are visible on speculum examination, then remove IUD to decrease the risk of miscarriage and infection;
- If the pregnancy is in the first trimester and desired, the pregnancy and IUC are intrauterine, but the strings are not visible, IUD removal may be performed under ultrasound guidance using alligator forceps or an IUD hook. Data on hysteroscopic removal of IUD in early pregnancy is very limited. If removal appears difficult, the IUD may also be left in situ.
- If the woman desires pregnancy termination, IUD removal can be performed at the time of the termination of pregnancy. If she is having a spontaneous abortion, remove the IUD and prescribe antibiotics (doxycyline 100 mg twice a day or ampicillin 500 mg four times a day for seven days).
- If pregnancy is diagnosed after the first trimester, obtain an ultrasound to determine the placental and IUD locations. The woman should be counseled that with the IUD in place, she is at increased risk of preterm labor and delivery (fourfold increase), second trimester fetal loss, and infection, but not at increased risk of birth defects. If the IUD is removed or expelled without complications, there is no increased risk of miscarriage. However, removal may cause rupture of membranes or bleeding and subsequent pregnancy loss. If the strings are visible and removal appears safe based upon ultrasound localization of the IUD, the device may be removed by pulling on the strings. If strings are not visible, the IUD may be left in situ.
Other Effects:There is no increase in incidence of abnormal cervical cytology attributable to intrauterine contraception in the literature.
Summary:
The modern intrauterine contraception (IUC) is a highly effective, safe, cost-effective, long-acting, and rapidly reversible method of contraception with few side effects. It is a private and convenient method of contraception, does not interfere with the spontaneity of sex, offers several non-contraceptive health benefits, and can be used with breastfeeding. Although multiparous and monogamous women have been traditional candidates for intrauterine devices (IUDs) in the United States, international and national guidelines support their wider use. Clinicians should consider intrauterine contraception in appropriate candidates, including nulliparous women, adolescents, women immediately postpartum or postabortal, women desiring emergency contraception, and as an alternative to permanent sterilization. Barriers to IUD use such as requiring pre-insertion testing in low-risk women or insertion only during menses should be removed. There are three categories of modern IUCs: copper IUCs, progestin-releasing IUCs, and unmedicated (inert) IUDs. The TCu380A IUC and the LNG-IUS are similarly effective, and more effective than inert IUDs. Advantages of the TCu380A IUC include that it can be used in women who want or need to avoid hormonal contraception, it may be left in place for as long as 10 years. The major disadvantage of the TCu380A is that menses become heavier (approximately 50% increase in blood loss) and sometimes more painful. The LNG-IUS is associated with significantly reduced menstrual blood loss, as well as decreased dysmenorrhea. Discontinuation is most commonly due to complaints of menstrual disturbances and complaints of systemic hormonal side effects. Ectopic pregnancy is rare in IUC users and less frequent than in women not using contraception. The risk of PID in not increased in IUC users after the period immediately following insertion. Women conceiving with an intrauterine device are at increased risk for adverse obstetric outcomes, whereas the risk is higher for pregnancies with retained intrauterine device compare with early intrauterine device removal.
Suggested Readings:
- World Health Organization
Long-term safety and effectiveness of copper-releasing intrauterine devices: a case-study - Centers for Disease Control and Prevention (CDC)
Update on intrauterine devices (IUD) and pelvic infection - National Institutes of Health (NIH)
Contraception
References:
- Trussell J. Contraceptive failure in the United States.Contraception 2004;70(2):89-96
- World contraceptive use 2001. United Nations Population Division, Department of Economic and Social Affairs, New York, 2002
- Making decisions about contraceptive introduction: a guide for conducting assessments to broaden contraceptive choice and improve quality of care. Geneva, World Health Organization, 2002 (WHO/RHR/02.08)
- O'Brien Pam Marfleet C. Frameless versus classical intrauterine device for contraception. Cochrane Database Syst Rev 2001; CD003282
- French R, Van Vliet H, Cowan F et al. Hormonally impregnated intrauterine systems (IUSs) versus other forms of reversible contraceptives as effective methods of preventing pregnancy. Cochrane Database Syst Rev 2004; CD001776
- Stanford JB, Mikolajczyk RT. Mechanism of action of intrauterine devices: update and estimation of post-fertilization effects. Am J Obstet Gynecol 2002;187:1677-1708. (Level III)
- El-Tagy A, Sakr E, Sokal DC et al. Safety and acceptability of post-abortal IUD insertion and the importance of counseling. Contraception 2003;67:229-236
- World Health Organization. Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: WHO; 2004
- Speroff L, Darney P. Intrauterine contraception: the IUD. In: Clinical gynecologic endocrinology and infertility. 7th edition, Lippincott Williams & Wilkins, Philadelphia 2005; p. 975-995
- Farley TM, Rosenberg MJ, Rowe PJ et al. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet 1992;339:785-788
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Intrauterine device. Number 59. Obstet Gynecol 2005;105:223-232
- Hubacher D, Lara-Ricalde R, Taylor DJ et al. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med 2001;345:561-567
- Merki-Feld GS, Lebeda E, Hogg B et al. The incidence of actinomyces-like organisms in Papanicolaou-stained smears of copper- and levonorgestrel-releasing intrauterine devices.Contraception 2000;61:365-368. (Level III)
- Bonacho I, Pita S, Gomez-Besterio MI. The importance of the removal of the intrauterine device in genital colonization by actinomyces. Gynecol Obstet Invest 2001;52:119-123. (Level I)
- Furlong LA. Ectopic pregnancy risk when contraception fails: A review. J Reprod Med 2002;47:881-890
- Backman T, Huhtala S, Blom T et al. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system: a nation-wide study of 17,360 users. BJOG 2000;107:335-342
- Grimes D, Schulz K, van Vliet H et al. Immediate post-partum insertion of intrauterine devices: a Cochrane review. Hum Reprod 2002;17:549-552
- Hatcher RA, Trussell J, Stewart F et al. Contraceptive Technology. 18th ed, Arden Media Inc., New York 2004
- Steiner MJ, Trussell J, Mehta N et al. Communicating contraceptive effectiveness: a randomized controlled trial to inform a World Health Organization family planning handbook. Am J Obstet Gynecol 2006;195(1): 85-91
- Weir E. Preventing pregnancy: a fresh look at the IUD.CMAJ 2003;169-585-591
- Grimes DA, Lopez LM, Manion C et al. Cochrane systematic reviews of IUD trials: lessons learned. Contraception 2007;75(6 Suppl.):S55-S59
- Gardner FJ, Konje JC, Bell SC et al. Prevention of tamoxifen induced endometrial polyps using a levonorgestrel releasing intrauterine system. Long-term follow-up of a randomized control trial. Gynecol Oncol 2009;114:452-456
- Browne H, Manipalviratn S, Armstrong A. Using an intrauterine device in immunocompromised women. Obstet Gynecol 2008;112:667-669
- Allen RH, Goldberg AB, Grimes DA. Expanding access to intrauterine contraception. Am J Obstet Gynecol 2009;201:456.e1-5
- Ganer H, Levy A, Ohel I et al. Pregnancy outcome in women with an intrauterine contraceptive device. Am J Obstet Gynecol 2009;201:381.e1-5
Published: 2 December 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com