Polycystic Ovary Syndrome
WHEC Practice Bulletin and Clinical Management Guidelines for healthcare providers. Educational grant provided by Women's Health and Education Center (WHEC).Polycystic ovary syndrome (PCOS) is a condition of unexplained chronic anovulation state. In 1935, Stein and Leventhal first described a symptom complex associated with anovulation. Acceptance of this syndrome as a singular clinical entity led to a rather rigid approach to this problem for many years. Only those women qualified who had a history of oligomenorrhea, hirsutism, and obesity together with a demonstration of enlarged polycystic ovaries. It is far more useful clinically to avoid the use of eponyms and even the term polycystic ovary syndrome or disease. It is better to consider this problem as one of persistent anovulation with a spectrum of etiologies and clinical manifestations. Recent findings suggest polycystic ovary syndrome (PCOS) has substantial metabolic sequelae, including risk of diabetes and possibly cardiovascular disease, and that primary treatment should focus on metabolic sequelae. Its etiology remains unknown, and treatment is largely symptom based and empirical.
The purpose of this document is to enhance understanding of the best available evidence on the diagnosis and clinical management of polycystic ovary syndrome (PCOS). A question which has puzzled gynecologists and endocrinologists for many years is what causes polycystic ovaries. The characteristic polycystic ovary emerges when a state of anovulation persists for any length of time. Whether diagnosis is by ultrasound or by the traditional clinical and biochemical criteria, a cross-section of anovulatory women at any one point of time will reveal that approximately 75% will have polycystic ovaries. Variety of treatments of PCOS is also discussed in this chapter and the healthcare providers must appreciate the clinical impact of anovulation and should undertake appropriate managements.
Etiology:
No gene or specific environmental substance has been identified as causing PCOS. Selective insulin resistance may be central to the etiology of the syndrome: skeletal muscle is profoundly resistant, and other tissues (hypothalamus, adrenal, and ovary) remain sensitive to the effects of insulin. Polycystic ovary is considered as a result of a functional derangement, not a specific central or local defect. In contrast to the characteristic picture of fluctuating hormone levels in the normal cycle, a "steady state" of gonadotropins and sex steroids can be depicted in association with persistent anovulation. This steady state is only relative, and is being exaggerated here to present a concept of this clinical problem. Compensatory increased levels of insulin may result in decreased levels of sex hormone binding globulin (SHBG) and serve as a trophic stimulus to androgen production in the adrenal gland and ovary (1). Obesity may be associated with three alterations that interfere with normal ovulation, and weight loss improves all three: increased peripheral aromatization of androgens to estrogens; decreased levels of sex hormone binding globulin (SHBG) resulting in increased levels of free estradiol and testosterone; increased insulin level that can stimulate ovarian stromal tissue production of androgens.
Definition and Diagnostic Criteria:
Although there is no universally accepted definition of PCOS, diagnostic criteria established by the National Institutes of Health in 1990 define it as hyperandrogenism, and chronic anovulation in cases in which secondary causes (such as adult-onset congenital adrenal hyperplasia, hyperprolactinemia, and androgen secreting neoplasms) have been excluded. Insulin resistance has been noted consistently among many women with unexplained hyperandrogenic chronic anovulation, but it is not included in the diagnostic criteria. Ultrasonograms of women with unexplained hyperandrogenic chronic anovulation frequently show ovaries that appear polycystic; however, polycystic ovaries are a non-specific finding and also are frequently noted in women with no endocrine or metabolic abnormalities. Hyperandrogenic chronic anovulation occurs in approximately 4-6% of women, with no significant differences in the prevalence of hirsutism or elevated circulating androgen levels between white and black women (2).
The prevalence of PCOS in women of reproductive age is approximately 5%, making it one of the most common reproductive disorders. Among women with ovulatory dysfunction, about 70% have PCOS. In the largest clinical trial to date of women of PCOS, 50-60% of the 400 women prospectively identified as having hyperandrogenic chronic anovulation, had no evidence of hirsutism. However, not all women with hirsutism will have androgen excess, and not all women with androgen excess will have hirsutism. Hyperandrogenism can be established on the basis of clinical findings (e.g., hirsutism or acne) or hormone measurement or both. The polycystic ovary is usually enlarged and is characterized by a smooth pearly white capsule. The characteristics of ovary reflect this dysfunction is: the surface area is doubled, giving an average volume increase of 2.8 times; the same number of primordial follicles is present, but the number of growing and atretic follicles (up to the secondary follicle stage) is doubled. Each ovary may contain 20-100 cystic follicles. The thickness of the tunica (outermost layer) is increased by 50%. A one-third increase in cortical stromal thickness and a 5-fold increase in subcortical stroma are noted. The increased stroma is due to both hyperplasia of thecal cells and to increased formation subsequent to the excessive follicular maturation and atresia. There are 4 times more ovarian hilus cell nests (hyperplasia).
n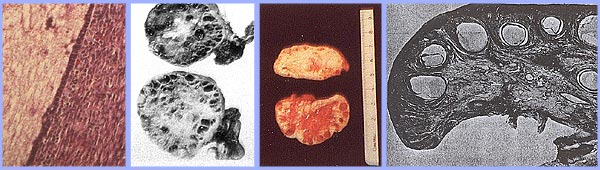
Polycystic ovary with a thickened capsule and prominent subcapsular cysts. Note lack of lutea or corpora albicantia due to anovulation.
Clinical Manifestations and Diagnosis:
Women with PCOS commonly present with infertility or menstrual disorders. In addition, women with PCOS appear to be at increased risk for complications of pregnancy, including gestational diabetes and hypertensive disorders; the risk of these complications is further exacerbated by multiple pregnancies (3). Chronic anovulation, obesity, hyperinsulinemia, and decreased levels of SHBG are all associated with endometrial cancer. Insulin resistance and its associated conditions, such as acanthosis nigricans, centripetal fat distribution, obesity, and obesity-related sleep disorders, are all common with PCOS. In turn, all of these risk factors for long-term metabolic sequelae, such as type II diabetes and cardiovascular disease. The physical examination should include an evaluation of balding, acne, clitoromegaly, and body hair distribution, as well as a pelvic examination to look for ovarian enlargement. Suggested diagnostic evaluation for PCOS are: Blood Pressure; Body Mass Index (BMI) (weight in kg divided by height in m2) (kg/m2), BMI of 25-30 = overweight, >30 = obese; Waist-hip ratio to determine body fat distribution, value >0.72 = abnormal; Presence of stigmata of hyperandrogenism or insulin resistance, such as, acne, hirsutism, androgenic alopecia, acanthosis nigricans.
Laboratory testing is to document hyperandrogenism (total testosterone and/or bioavailable or free testosterone) and exclusion of other causes of hyperandrogenism -- thyroid stimulating hormone levels (thyroid function); prolactin levels (hyperprolactinemia); 17-Hydroxyprogestrone (nonclassical congenital adrenal hyperplasia caused by 21-hydroxylase deficiency): random normal level < 4 ng/ml or morning fasting level < 2 ng/ml. Because Cushing's syndrome is extremely rare (1 in 1,000,000) and screening tests are not 100% sensitive or specific, routine screening for Cushing's syndrome in all women with hyperandrogenic chronic anovulation is not indicated. Those who have co-existing signs of Cushing's syndrome, including a moon face, buffalo hump, abdominal striae, centripetal fat distribution, or hypertension, should be screened (4). Evaluation for metabolic abnormalities: 2-hour oral glucose tolerance test (fasting glucose < 110 mg/dl = normal; 110-125 mg/dl = impaired; >126 mg/dl = type II diabetes) followed by 75 g oral glucose ingestion and then 2 hour glucose level (<140 mg/dl = normal glucose tolerance, 140-199 mg/dl = impaired glucose tolerance, >200 mg/dl = type II diabetes). Fasting lipid and lipoprotein level (total cholesterol, high-density lipoprotein, triglycerides) should also be considered. Other optional tests to consider are: gonadotropins determinations to determine cause of amenorrhea, fasting insulin levels in younger women, those with severe stigmata of insulin resistance and hyperandrogenism, or those undergoing ovulation induction. 24-hour urine test for urinary free cortisol with late onset of polycystic ovary syndrome symptoms or stigmata of Cushing's syndrome are helpful.
Androgen-secreting tumors of the ovary or adrenal gland are invariably accompanied by elevated circulating androgen level. However, there is no absolute level that is pathognomic for a tumor, just as there is no minimum androgen level that excludes a tumor. Evaluation of DHEAS levels may be useful in cases of rapid virilization (as a marker of adrenal origin), but its utility in assessing common hirsutism is questionable. Mild elevation of prolactin level in women with PCOS is common. A prolactinomas that secrete large amounts of prolactin and that may stimulate ovarian androgen production, but this is an extremely rare cause of hyperandrogenic chronic anovulation (5). Evaluating serum levels of thyroid stimulating hormone also is useful given the protean manifestations and frequency of thyroid disease in women.
Ultrasound examination of ovaries for baseline evaluation and morphology before ovulation induction or in cases of virilization or rapid conversion to an androgen excess state is very helpful.
n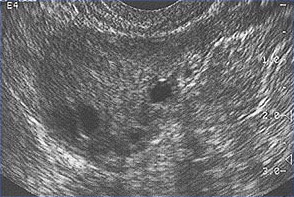
This image shows ovarian follicles in a woman with regular ovulatory cycles
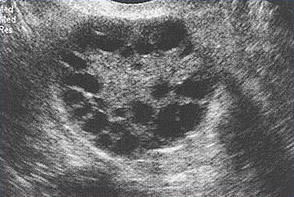
Multiple small follicles are shown here in a woman with PCOS.
Management:
The clinical consequences of persistent anovulation are: dysfunctional uterine bleeding; amenorrhea; infertility; hirsutism; acne; increased risk of endometrial cancer and perhaps breast cancer; increased risk of cardiovascular disease; and increased risk of diabetes mellitus.
Polycystic ovary disease (PCOS) with dysfunctional uterine bleeding not attempting to conceive:
- Combination Oral Contraceptives -- it has been mainstay to regulate menstrual cycles. They offer benefit through a variety of mechanisms, including suppression of pituitary luteinizing hormone secretion, suppression of ovarian androgen secretion, and increased circulating SHBG. The "best" oral contraceptive for women with PCOS is not known. Oral contraceptives also are associated with a significant reduction in the risk for endometrial cancer, but the magnitude of the effect in women with PCOS is not known (6).
- Progestin -- both depot and intermittent oral medroxyprogesterone acetate (10 mg for 10 days) have been shown to suppress pituitary gonadotropins and circulating androgens in women with PCOS. Use of medroxyprogesterone acetate has been associated with decreases in SHBG in women with PCOS. Progestin only oral contraceptives are an alternative for endometrial protection, but they are associated with a high incidence of breakthrough bleeding.
- Insulin-Sensitizing Agents -- these agents are: biguanides (metformin), thiazolidinediones (troglitazone, pioglitazone, and rosiglitazone), and an experimental insulin sensitizer drug known as D-chiro-inositol. They do not increase insulin secretion, as do sulfonylureas, and are thus rarely associated with hypoglycemia, a risk for those who are normoglycemic when fasting (as are most women with PCOS). There are class differences, for example, biguanides tend to decrease weight and thiazolidinediones to increase weight. It is difficult to separate the effects of improving insulin sensitivity from those of lowering serum androgens, as any "pure" improvement in insulin sensitivity can increase SHBG and thus decrease bioavailable androgen (7). None of the agents noted are currently approved by the U.S. Food and Drug Administration (FDA) for treatment of PCOS. Despite encouraging preliminary results, troglitazone was removed from the world wide market because of hepatotoxicity.
Polycystic ovary syndrome (PCOS) with Infertility:
There is no evidence-based schema to guide the initial and subsequent choices of ovulation induction methods in women with PCOS. Treatment should begin with a regimen of regular exercise and weight control and then proceed to other methods if necessary. Most commonly use agents to induce ovulation are:
- Clomiphene citrate -- this has traditionally been the first-line treatment agent for anovulation. Up to 80% or women with PCOS will ovulate in response to clomiphene treatment, and 50% of these women will conceive. One half of all women who are going to conceive using clomiphene will do so with the 50-mg starting dose, and another 20% will do so with the 100-mg per day dosage. Most pregnancies occur within the first six ovulatory cycles. Increasing the duration of treatment adds little to the pregnancy rate. Alternative clomiphene regimens have been developed, including prolonging the period of administration and adding dexamethasone. Dexamethasone as adjunctive therapy with clomiphene citrate has been shown to increase ovulation rates in women with PCOS with higher DHEAS levels (>2,000 ng/mL) (8).
- Gonadotropins -- they are frequently used to induce ovulation in women with PCOS for whom clomiphene treatment has failed. Low-dose therapy with gonadotropins offers a higher rate of monofollicular development (approximately 50% or greater) with a significantly lower risk of ovarian hyperstimulation syndrome (20-25%) that results in cycle cancellation or more serious sequelae (9).
- Metformin -- insulin sensitizing agent has shown that it improves ovulatory frequency in women with PCOS. The dosage most frequently used has been 1,500 mg per day, and more recent studies have used 2,000 mg per day in divided doses. Metformin also has been used successfully as an adjunctive agent with both clomiphene citrate and gonadotropins. Metformin carries a small risk of lactic acidosis, most commonly among women with poorly controlled diabetes and impaired renal function. Gastrointestinal symptoms (diarrhea, nausea, vomiting, abdominal bloating, flatulence, and anorexia) are the most common adverse reactions and may be ameliorated by starting at a small dose and gradually increasing the dose or by using the sustained-release version now available in the United States. Metformin has no known human teratogenic risk or embryonic lethality in humans and appears safe in pregnancy. Some clinicians advocate its use during early pregnancy to reduce the miscarriage rate, but the documentation for this claim is poor (10).
- Thiazolidinediones -- they are peroxisome proliferators activating receptor (PPAR-y) agonists and are thought to improve insulin sensitivity through a post receptor mechanism. It is effective in improving ovulation and hirsutism. These benefits appear to be mediated through decreases in hyperinsulinemia and decreases in free testosterone levels (with corresponding increases in SHGB).
Ovarian Drilling: the value of laparoscopic ovarian drilling with laser or diathermy as a primary treatment for sub-fertile women with anovulation and PCOS is undetermined. Neither drilling by laser nor diathermy has any obvious advantages, and there is insufficient evidence to suggest a difference in ovulation or pregnancy rates when drilling is compared with gonadotropins therapy as a secondary treatment for women who do not respond to clomiphene. Multiple pregnancy rates are reduced in those women who conceive after laparoscopic drilling. In some cases, the fertility benefits of ovarian drilling may be temporary, and drilling does not appear to improve metabolic abnormalities in women with PCOS (11).
Step-by-Step Approach to Ovulation Induction in Women with PCOS:
The least resource-intensive interventions are recommended in the early steps in the protocol, while the most resource-intensive interventions are reserve for later treatment (12);
Step 1: If the BMI is higher than 30, recommend weight loss of at least 10% of body weight.
Step 2: Prescribe clomiphene to induce ovulation.
Step 3: If DHEAS is higher than 2 micro g/mL, consider combining clomiphene with a glucocorticoid to induce ovulation.
Step 4: If clomiphene does not result in ovulation, consider a combination of metformin plus clomiphene.
Step 5: Initiate low-dose FSH injections.
Step 6: Initiate low-dose FSH injections plus metformin.
Step 7: Consider laparoscopic ovarian surgery (drilling) or in-vitro fertilization (IVF).
Polycystic Ovaries and Uterine Fibroids:
The lack of a detrimental effect of PCOS on the uterine artery blood flow in women >35 years old tallies with the decreased hyperandrogenization and tendency toward more regular cycles reported in older women with PCOS. It could be hemodynamic reflection of such improved ovarian biochemistry and function in this age group. Furthermore, the fact that fewer patients with PCOS had uterine fibroids in many studies than those with normal ovaries has not been previously reported. This finding contradicts a study that involved 21-69 year-old African American women utilizing post questionnaires for recruiting patients (13). The difference could reflect the two different age group ranges and the methodologies used in the two studies. The negative correlation between the presence of PCOS and fibroids and the lack of significant association between age and fibroids in women with PCOS in this study suggested a protective role for PCOS in that respect. It was probable that lower myometrial blood perfusion in women with PCOS, as shown in this and other studies, was a contributing factor in reducing their chances of developing fibroids. As well, the hyperadrogenic tendency with PCOS even in women with regular menstrual cycles might have had a protective effect on the uterine muscles as fibroids usually grow more readily in an estrogenic environment. This study (14) showed that patients with PCOS are less likely to have uterine fibroids. The detrimental effect of PCOS on the uterine blood flow in women with regular menstrual cycles added a hemodynamic aspect to the endocrine abnormalities previously reported in similar groups of patients. Patient's age and parity modulated the effects of PCOS and fibroids on the uterine artery blood flow. Accordingly, any statement relating uterine blood flow to the presence or absence of PCOS or fibroids should take these two factors into consideration.
In obese women with PCOS, does weight loss improve ovarian function?
Obesity contributes substantially to reproductive and metabolic abnormalities in women with PCOS. Multiple studies have shown that weight loss can improve the fundamental aspects of the endocrine syndrome of PCOS by lowering circulating androgen levels and causing spontaneous resumption of menses. Reduction in body weight has been associated with improved pregnancy rates and decreased hirsutism, as well as improvements in glucose and lipid levels. Studies using pharmacologic weight loss agents, such as orlistat, and intestinal inhibitor of lipid absorption, and sibutramine, and anorexic agent, in women with PCOS have shown similar improvement in ovarian function (15). Morbidly obese women with PCOS who undergo bypass surgery experience near normalization of their reproductive and metabolic abnormalities. These changes have been reported with weight loss as little as 5% of the initial weight. The decrease in unbound testosterone levels after weight loss may be largely mediated through increases in SHBG (16). The effects of weight loss in normal weight women with PCOS are unknown.
Summary:
Polycystic ovary syndrome is defined as the presence of oligomenorrhea or amenorrhea and hyperandrogenism in the absence of other hyperandrogenic disorders, such as androgen-secreting tumors or nonclassical adrenal hyperplasia. Clinical evidence of hyperandrogenism includes hirsutism and acne. Laboratory evidence of hyperandrogenism includes an elevated total, bioavailable, or free testosterone concentration. Elevated serum dehydroepiandrosterone sulfate (DHEAS) or androstenedione levels also are evidence of hyperandrogenism. The morphologic characteristics of "polycystic ovaries" as demonstrated on pelvic ultrasonography are not essential for the diagnosis of PCOS but support the diagnosis. In women with PCOS, many therapies are available to treat anovulatory infertility, including weight loss, clomiphene, clomiphene plus metformin, clomiphene plus glucocorticoid, gonadotropins injections, ovarian surgery, and in vitro fertilization-embryo transfer (IVF-ET).
All women with PCOS should be screened for glucose intolerance with a 2-hour glucose level after a 75 fasting glucose challenge. Interventions that improve insulin sensitivity, including weight loss, use of metformin, and use of thiazolidinediones, are useful in improving ovulatory frequency in women with PCOS. Use of clomiphene citrate is appropriate because it effectively results in pregnancy in women with PCOS. Improvements in insulin sensitivity, by weight loss or by the use of insulin-sensitizing agents, may favorably improve many risk factors for diabetes and cardiovascular disease in women with POCS. When using gonadotropins to induce, low-dose therapy is recommended because it offers a high-rate of monofollicular development and a significantly lower risk of ovarian hyperstimulation in women with PCOS. The benefit and role of surgical therapy in ovulation induction in women with PCOS is uncertain. The best or initial treatment for hirsutism, ovulation induction, or prevention of long-term metabolic sequelae for women with PCOS is unknown. All of these conditions may benefit from lifestyle modification.
References:
- Azziz R, Ehrmann D, Legro RS et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab 2001;86:1626-1632. (Level I)
- ACOG Practice Bulletin. Polycystic ovary syndrome. Number 108, October 2009
- Chang RJ. A practical approach to the diagnosis of polycystic ovary syndrome. Am J Obstet Gynecol 2004;191:713-717
- Park JK, Loucks TL, Berga SL. Polycystic ovary syndrome. In: Falcone T, Hurd WW eds. Clinical reproductive medicine and surgery. New York: Elsevier; 2007. p. 217-232
- Kolodziejczyk B, Duleba AJ, Spaczynski RZ et al. Metformin therapy decreases hyperandrogenism and hyperinsulinemia in women with polycystic ovary syndrome. Fertil Steril 2000;73:1149-1154
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril 2008;89:505-522
- Chang PL, Lindheim SR, Lowre C et al. Normal ovulatory women with polycystic ovaries have hyperadrogenic pituitary-ovarian responses to gonadotropin-releasing hormone agonist testing. J Clin Endocrinol Metab 2000;85:995-1000
- Glueck CJ, Philips H, Cameron D et al. Continuing metformin throughout pregnancy in women with polycystic ovary syndrome appears to safely reduce first-trimester spontaneous abortion: a pilot study. Fertil Steril 2001;75:46-52 (Level III)
- Farquhar CM, Willamson K, Gudex G et al. A randomized controlled trial of laparoscopic ovarian diathermy versus gonadotropins therapy for women with clomiphene citrate-resistant polycystic ovary syndrome. Fertil Steril 2002;78:404-411. (Level I)
- La Marca A, Artensio AC, Stabile G et al. Metformin treatment of PCOS during adolescence and the reproductive period. Eur J Obstet Gynecol Reprod Biol 2005;121:3-7
- Zain MM, Jamaluddin R, Ibrahim A et al. Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: a randomized controlled trial. Fertil Steril 2009;91:514-521. (Level I)
- Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med 2008;358:47-54
- Wise L, Palmer J, Stewart E et al. Polycystic ovary syndrome and risk of uterine leiomyomata. Fertil Steril 2007;87:1108-1118
- Abdel-Gadir A, Oyawoye OO, Chander BP. Coexistence of polycystic ovaries and fibroids and their combined effect on the uterine artery blood flow in relation to age and parity. J Reprod Med 2009;54:347-352
- Lindholm A, Bixo M, Bjorn I et al. Effect of sibutramine on weight reduction in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Fertil Steril 2008;89:1221-1228. (Level I)
- ACOG Practice Bulletin. Polycystic ovary syndrome. Number 108, October 2009. Obstet Gynecol 2009;114:936-949
Published: 19 November 2009
Dedicated to Women's and Children's Well-being and Health Care Worldwide
www.womenshealthsection.com


